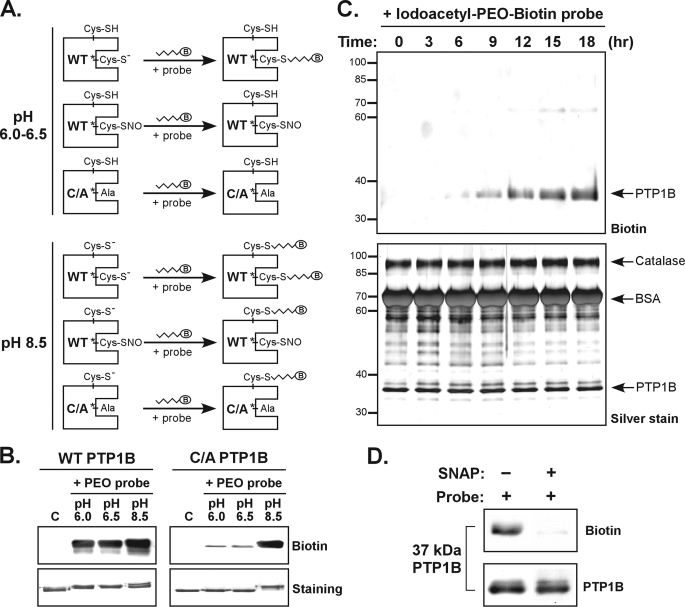
FIGURE 2.
The PEO probe recognizes the reduced form but not the S-nitrosylated form of PTP1B. A, schematic illustration of the PTP labeling workflow used to determine a condition for specific biotinylation of the active site Cys. The WT or C215A (C/A) mutant form of recombinant PTP1B was used in these experiments. Probes tested contain a biotin group (indicated as B in an oval). B, the Iodoacetyl-PEO-Biotin probe (PEO probe) was used for the analysis. The lanes C indicate control samples in which PTP1B was diluted in buffer (pH 7.5) without probe. For labeling reaction, the PEO probe (2 mm) was incubated with an aliquot of PTP1B (2 μg) at 25 °C for 90 min. The reaction was terminated by the addition of SDS-PAGE sample buffer followed by boiling for 6 min. An aliquot of PTP1B was subjected to immunoblotting with anti-biotin antibody or SDS-PAGE for staining with Coomassie Blue. C, the WT PTP1B (10 μg) was mixed with purified catalase (30 μg) and BSA (300 μg). This protein mixture was incubated with 1 mm PEO probe in a reaction buffer (pH 6.0) at 4 °C. At various times as indicated, an aliquot of protein mixture was harvested, mixed with SDS-PAGE sample buffer, and boiled. The samples were subjected to immunoblotting with anti-biotin antibody (upper panel), or SDS-PAGE for silver staining (lower panel). The arrowheads indicate the positions of proteins appearing in SDS-PAGE gels. D, the WT PTP1B (2 μg) was preincubated with 20 μm DTT for 10 min at 37 °C. The protein was left in the buffer containing DTT, or treated with the NO donor S-nitroso-N-penicillamine (SNAP, 1 mm) for 20 min at 37 °C. After removal of excessive DTT and S-nitroso-N-penicillamine by a PD-10 column, PTP1B was reconstituted in an acidic buffer (pH 6.0) and then incubated with the PEO probe (1 mm) for 30 min at 25 °C. An aliquot (375 ng) of PTP1B was subjected to immunoblotting with antibodies to biotin and PTP1B.