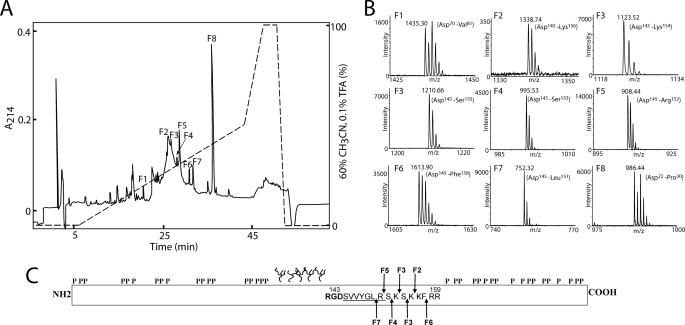
FIGURE 2.
Reversed-phase HPLC separation and MS analysis of N-terminal OPN digested with Asp-N in a buffer containing 50% H218O. A, peptides were separated on a narrow-bore reversed-phase chromatography C2/C18 PC 2.1/10 column operated by a SMART system (GE Healthcare). Separation was carried out in 0.1% trifluoroacetic acid (TFA), and peptides were eluted with a gradient of 60% acetonitrile in 0.1% trifluoroacetic acid (dashed line) at a flow rate of 0.15 ml/min. The peptides were detected in the effluent by measuring the absorbance at 214 nm (solid line). B, all fractions were analyzed by MS, and representative data from fractions F1 to F8 are shown. Only the relevant m/z region is shown. In fractions F1 and F8, two internal peptides are identified based on the characteristic isotopic pattern caused by incorporation of 18O. Peptides present in fractions F2–F7 were identified as C-terminal peptides as these retain their natural isotopic distribution. C, schematic representation of OPN showing the identified cleavage sites in milk OPN. The location of phosphorylations and O-glycosylations is based on the post-translational modification pattern from human milk OPN (31). The RGD sequence is shown in boldface, and the cryptic integrin-binding site SVVYGLR is underlined. The cleavage sites identified in fraction F2–F7 are shown with arrows and are all located in the near vicinity of the integrin-binding motifs.