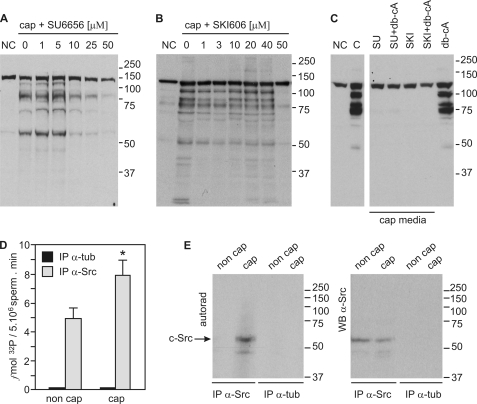
FIGURE 1.
SFK inhibitors abrogate tyrosine phosphorylation associated with sperm capacitation. A, mouse sperm were incubated in the absence or in the presence of SU6656 for 60 min in capacitating (cap, with HCO3−) or non-capacitating media (NC, without HCO3−). Western blot analyses were performed with anti-pY antibodies. B, sperm were treated and processed as in panel A in the absence or in the presence of SKI606 (bosutinib). C, sperm were incubated for 60 min in capacitating medium containing 50 μm of either SU6656 or SKI606 in the presence or absence of 1 mm Bt2cAMP/0.1 mm IBMX (db-cA). Tyrosine phosphorylation was assessed as in A. D, c-Src immunokinase assay using a synthetic peptide derived from cdc2 as substrate. Capacitated and non-capacitated sperm were extracted with radioimmune precipitation assay buffer and immunoprecipitated with anti-Src (clone GD11) antibodies or anti-tubulin as controls. Kinase assays were performed on the immunocomplexes. Data are represented as the mean ± S.E. of three independent experiments, each performed in triplicate; *, p < 0.05. E, kinase reaction mixtures containing the immunocomplexes were subjected to 8% SDS-PAGE, transferred to PVDF, and developed by autoradiography. Only c-Src from capacitated sperm displayed autophosphorylation activity (left panel, arrow). The same PVDF membrane was probed with anti-Src antibodies (clone 36D10). All Western blots are representative of experiments repeated at least three times.