FIGURE 2.
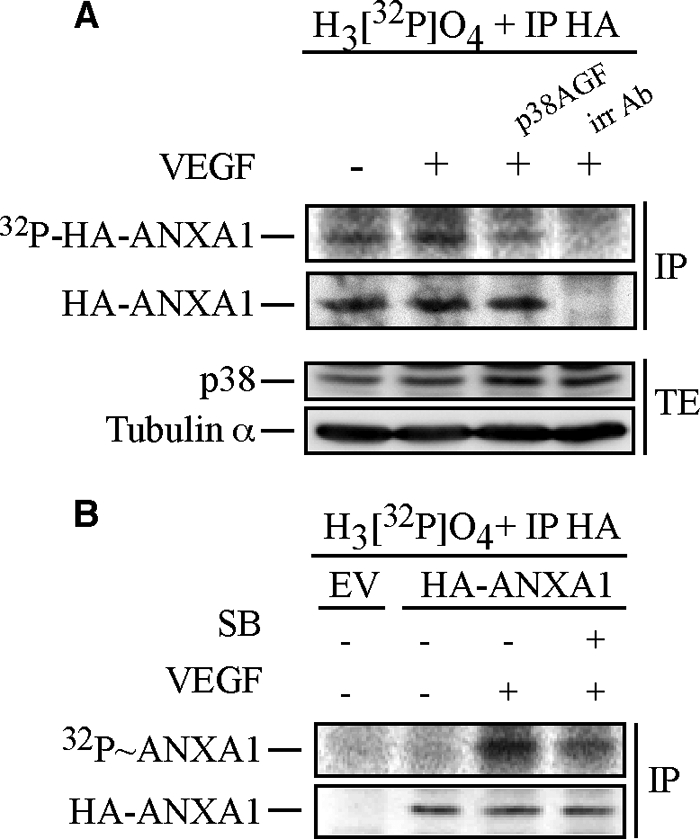
Annexin 1 is phosphorylated via the p38 pathway in response to VEGF. A, HUVECs were co-electroporated with pIRES-hrGFP-2a containing HA-tagged wild-type ANXA1 together with empty vector (EV) or pCMV carrying p38AGF. After 24 h, cells were serum-starved for 16–20 h. Then, quiescent HUVECs were treated or not treated with 5 ng/ml of VEGF for 15 min. Cells were extracted as described under “Experimental Procedures”. The HA-tagged proteins were immunoprecipitated (IP) using anti-HA mouse antibody. Immunoprecipitation using a mouse γ globulin irrelevant antibody (irr Ab) shows the specificity of the anti-HA antibody. Proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane, and the HA-ANXA1 band was analyzed using PhosphorImager. Thereafter, the membrane was processed for immunodetection of immunoprecipitated HA-ANXA1. Total proteins were kept before immunoprecipitation to monitor p38AGF expression. Tubulin α is also shown as a loading control. B, quiescent HUVECs transiently expressing an empty vector (EV) or HA-tagged wild-type ANXA1 were processed and treated as described in Fig. 1B. Cells were extracted as described under “Experimental Procedures.” After extraction, the HA-tagged proteins were immunoprecipitated using anti-HA mouse antibody. Proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was then exposed for autoradiography, analyzed using PhosphorImager, and processed for immunodetection of immunoprecipitated HA-ANXA1. Representative blots of at least two separate experiments are shown in A and B. SB, SB203580. TE, total extract.
