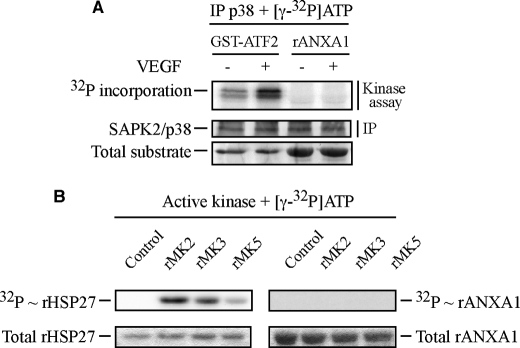
FIGURE 3.
p38 and its downstream kinases, MAPKAP kinase-2, MAPKAP kinase-3, and MAPKAP kinase-5, do not phosphorylate annexin 1 in response to VEGF. A, quiescent HUVECs were left untreated or stimulated with VEGF (5 ng/ml for 5 min). After treatments, cells were lysed, and p38 was immunoprecipitated (IP) using rabbit polyclonal antibody and subjected to an in vitro kinase assay. Reaction mixtures for kinase assay were put in the presence of [γ-32P]ATP. p38 activity was determined in immunocomplex assays using 2 μg recombinant GST-ATF2 as control substrate or 5 μg rANXA1. Protein mixtures were separated through SDS-PAGE and were transferred to a nitrocellulose membrane. Kinase activity was then quantified by autoradiography using the PhosphorImager system by measuring [32P] incorporation into the specific substrates. Membrane was also processed for immunodetection of immunoprecipitated p38. Representative results from three distinct experiments, each realized in duplicates, are shown. Total recombinant GST-ATF2 and total rANXA1 are shown as internal controls for the amount of added substrates. B, commercial purified and activated recombinant MAPKAP kinase-2 (rMK2), MAPKAP kinase-3 (rMK3) and MAPKAP kinase-5 (rMK5) were used in an in vitro kinase assay. As in A, reaction mixtures for kinase assays were put in the presence of [γ-32P]ATP, and kinase activities were determined using 1 μg of rHSP27 as a control substrate and 5 μg rANXA1. Protein mixtures were separated through SDS-PAGE. Kinase activities were then quantified by autoradiography using PhosphorImager. Total rHSP27 and total rANXA1 are shown as internal controls for the amount of added substrates. Control tracks containing all kinase assay compounds, except activated kinases, are also shown. Representative results of three separate experiments are shown.