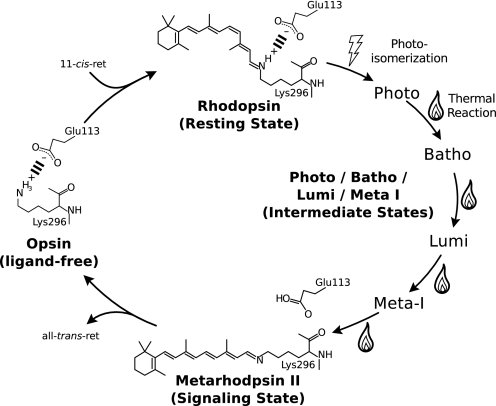
FIGURE 1.
The photocycle of bovine rhodopsin. In the ligand-free opsin state, the rhodopsin basal activity is diminished by the ionic pairing, called the opsin lock, a salt bridge formed from the positive charge of Lys-296 and the negative charge of Glu-113 (6, 7). The 11-cis-retinal chromophore functions as an inverse agonist and further suppresses the activity of the receptor, until photon absorption causes stereospecific isomerization of the retinal to its all-trans form and initiates a chain of structural changes (thermal reaction) that form the signaling state. The signaling state is thermally unstable, and the retinal is eventually expelled from the receptor, which quickly reforms the opsin lock to shut off the receptor.