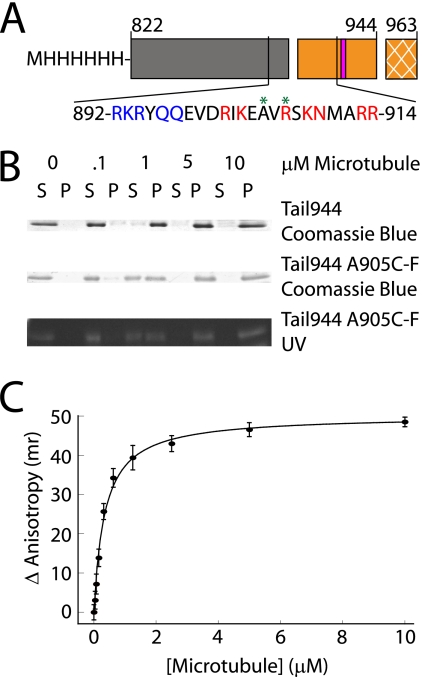
FIGURE 1.
A kinesin-1 tail construct binds to microtubules with a submicromolar affinity. A schematic representation of the tail constructs used in this work is shown in A. The regions of the tail are indicated as follows: coiled-coil (gray box), microtubule-binding and regulatory domain (orange box), and the extreme 19 C-terminal residues not included in these tail constructs (cross-hatched orange box). Residues (919–922) are critical for regulation of enzymatic activity, and are indicated by a pink bar. The amino acid sequence of the putative microtubule-binding site in the tail is also shown. Residues mutated to alanine in Mutant A are highlighted in blue, residues mutated to alanine in Mutant B are highlighted in red, and sites where fluorescein was attached are indicated with a green asterisk. Gels used to measure the affinity of Tail944 or Tail944 R907C-fluorescein (F) for microtubules by co-sedimentation are shown in B (S = supernatant or unbound tail, P = pellet or bound tail). The Tail944 R907C-F gel was also examined under UV light to demonstrate the specificity of the fluorescein labeling. Fluorescence anisotropy data for determining the affinity of Tail944 R907C-F for microtubules is shown in C. Kd values from both assays are reported in Table 1. Fluorescence anisotropy data are reported as the mean ± S.E. for three experiments.