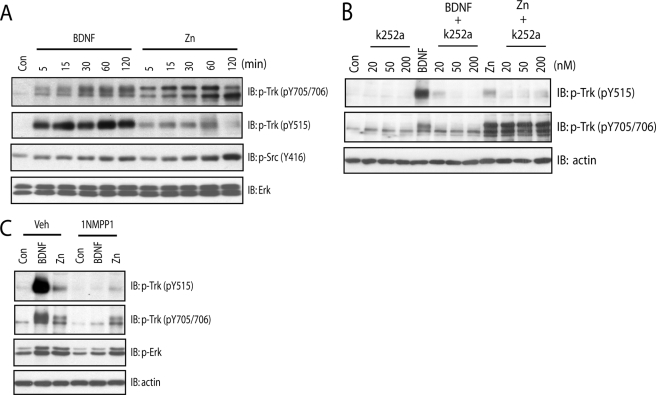
FIGURE 1.
Zinc increases phosphorylation of Tyr-705/Tyr-706 in a TrkB kinase-independent mechanism. In all of the experiments below, E18 rat (A and B) or P0-P1 mouse (C) cortical neurons were maintained in vitro for 12–14 days. Cell lysates were subjected to immunoblotting with the indicated antibodies after the treatments described. A, exogenous zinc induced preferential phosphorylation of Tyr-705/Tyr-706 in a time-dependent manner. Cortical neurons were incubated with vehicle, BDNF (10 ng/ml), or zinc (100 μm) for the indicated periods of time. B, the small molecule Trk inhibitor K252a reduced zinc-induced phosphorylation of Tyr-515 but not Tyr-705/Tyr-706. Cortical neurons were preincubated with vehicle or indicated concentrations of K252a for 15 min; each of these treatments was continued for an additional 15 min in the presence of vehicle, BDNF (10 ng/ml), or zinc (100 μm). C, the small molecule Trk inhibitor 1NMPP reduced zinc-induced phosphorylation of Tyr-515 but not Tyr-705/Tyr-706. Cortical neurons cultured from TrkBF616A mice were preincubated with vehicle or 1NMPP1 (1 μm) for 15 min; each of these treatments was continued for an additional 15 min in the presence of vehicle, BDNF (10 ng/ml), or zinc (100 μm).