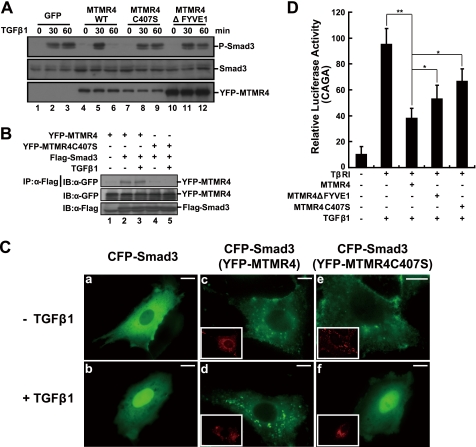
FIGURE 5.
The phosphatase catalytic pocket and the FYVE domain of MTMR4 are both critical for TGF-β signaling regulation. A, MTMR4 phosphatase-dead mutant C407S or FYVE domain deletion mutant fails to dephosphorylate activated Smad3. HeLa cells expressing control vector (3.75 μg, lanes 1–3), YFP-MTMR4 (3.75 μg, lanes 4–6), YFP-MTMR4-C407S (3.75 μg, lanes 7–9), or YFP-MTMR4ΔFYVE1 (3.75 μg, lanes 10–12) were treated with or without TGF-β1 for the times indicated. Endogenous activated Smad3 was immunoblotted with an anti-phosphor-Smad3 antibody (top panel). Protein loading of Smad3, YFP-MTMR4, and different mutants was verified by immunoblotting with anti-Smad3 antibody (middle panel) or anti-GFP antibody (bottom panel), respectively. B, phosphatase catalytic pocket of MTMR4 is in contact with Smad3. 293T cells were transiently cotransfected with pYFP-MTMR4 or pYFP-MTMR4-C407S (1.5 μg) and Flag-tagged Smad3 (1 μg) for 36 h. Cell lysates were immunoprecipitated with anti-Flag antibody, and blotted with anti-GFP antibody (top). Expression levels of YFP-MTMR4 (middle) and Smad3 (bottom) were also shown. C, MTMR4 sequesters Smad3 in early endosomes. PAE cells were transiently cotransfected with pCFP-Smad3 (green) and pYFP-MTMR4 or pYFP-MTMR4-C407S (red, insets) for 36 h. The subcellular localization of Smad3 and MTMR4 was detected using GFP and YFP channels, respectively, before and after TGF-β1 treatment. D, MTMR4 phosphatase-dead mutant and the FYVE domain deletion mutant fail to suppress TGFβ-induced 3TP-luciferase reporter activities efficiently. L17 cells reconstituted with TβRI were transiently cotransfected with CAGA reporter plasmid, and pCMV-hRL and either pYFP-MTMR4 30 ng, YFP-MTMR4-C407S 30 ng, or YFP-MTMR4ΔFYVE1 30 ng for 36 h. Luciferase assays were performed as described in Fig. 1C. **, p < 0.01; *, p < 0.05. The scale bar indicates 10 μm.