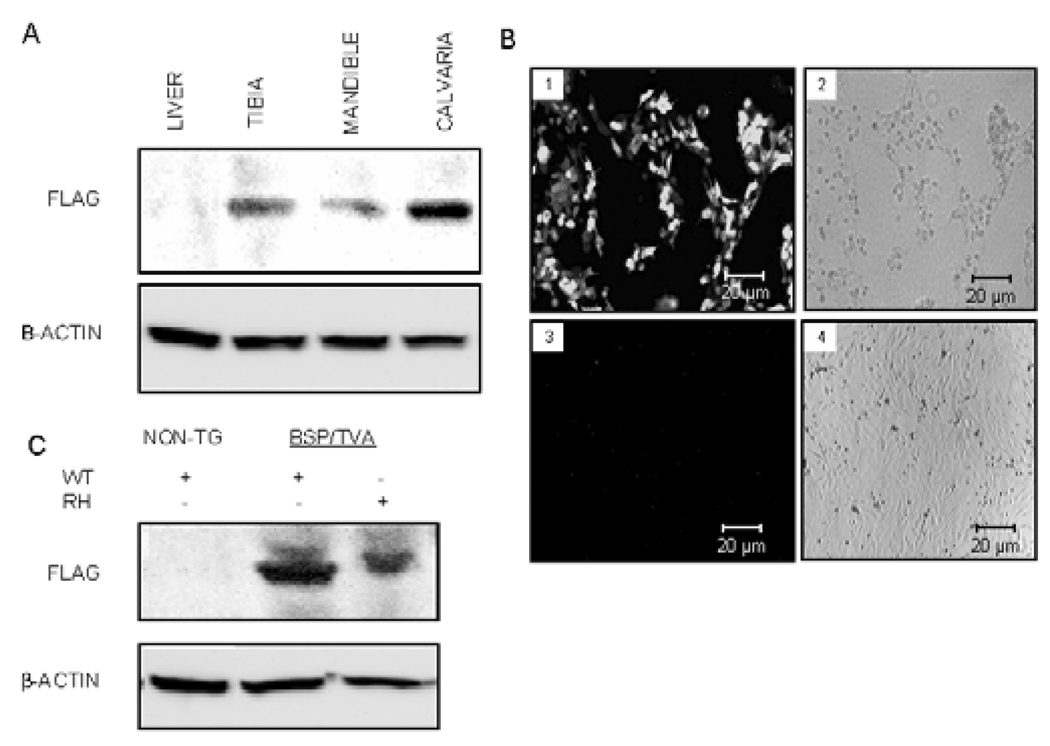
Figure 1.
BSP/TVA-driven Dlx5 or mutated Dlx5 overexpression in mineralized tissues and calvarial cultures. Western blot analyses were performed with tissues from BSP/TVA mice 4 days after infection with RCAS-Dlx5 (i.e., on day 9) and calvarial cells 3 days after in vitro infection with RCAS-Dlx5 or RCAS-Dlx5RH. (A) Expression analysis upon in vivo delivery of Dlx5 viral constructs. BSP/TVA mice were infected with RCAS-Dlx5, and expression of overexpressed Dlx5 was detected with an anti-FLAG antibody on day 9. An antibody for β-actin was used as a loading control. Protein lysates were obtained from tibial, mandibular, and calvarial tissues isolated from BSP/TVA mice infected with the RCAS-Dlx5 construct and from a representative soft tissue (i.e., liver). (B) GFP expression analyses upon ex vivo infection of RCAS-GFP into calvarial cells from BSP/TVA transgenic and non-transgenic mice. Calvarial cells from BSP/TVA mice (1,2) or wild-type mice that did not express TVA (2,4) were transfected with the RCASGFP construct. We used GFP expression to titer infection efficiency and to confirm the specificity of the overexpression to cells that express TVA 3 days after the infection. The in vitro-infected cells were photographed by fluorescence (1,3) and phase contrast (2,4) microscopy. Green fluorescence was detected in infected cells from BSP/TVA mice (1), but not in those of normal mice, due to the lack of TVA expression (3). (C) Western blot analysis upon ex vivo delivery of viral constructs. The analysis was performed with protein lysates from calvarial cells derived from non-transgenic mice (without TVA expression) before and after being infected ex vivo with RCAS-Dlx5. Calvarial cells from BSP/TVA mice were infected ex vivo with RCAS-Dlx5WT or RCAS-Dlx5RH viral constructs. Expression of exogenous Dlx5 constructs and the loading control β-actin were detected as described above.