Abstract
Studies of the role of individual genes in chronic lymphocytic leukemia (CLL) have been hampered by the inability to consistently transfect primary tumor cells. Here, we describe a highly efficient method of genetically modifying primary CLL cells using a VSVG pseudotyped lentiviral vector. We transduced CD38 negative CLL cells with a lentiviral vector encoding CD38 which caused increased surface CD38 expression in all the samples tested (n=17) with no evidence of plasmacytoid differentiation. The mean percentage of positive cells expressing CD38 was 87%±8.5% and the mean cell viability 74%±17%. This high level of transduction of all the CLL cell samples tested demonstrates the utility of this technique which should prove applicable for the introduction and analysis of other genes in these non-dividing cells.
Keywords: chronic lymphocytic leukemia, primary tumor cells, CD38 negative
Introduction
Chronic lymphocytic leukemia (CLL) is a heterogeneous disease ranging from a stable condition requiring no therapy to a progressive disease refractory to treatment. One important molecule associated with this disease is CD38: the expression of the CD38 antigen on the surface of clonal B cells is associated with a poor prognosis and reduced overall survival in patients with CLL.1–6 The expression of CD38 defines an altered pattern of gene expression including increased levels of anti-apoptotic, pro-inflammatory, signaling and pro-angiogenic molecules.7,8 However, these experiments were performed by comparing cells from different patients, with heterogeneous genetic backgrounds, and other studies depend on correlations in expression. These comparative experiments demonstrated a technical limitation of our ability to genetically modify CLL cells to alter CD38 expression.
To date, CLL cells have been difficult to genetically modify. The cells do not grow in liquid culture and most methods of manipulation result in the modification of a subset of cells and often cause substantial cell death. To address this problem, we developed a method of genetically modifying CLL cells using lentiviruses. This allowed us to increase CD38 expression in all the patient samples tested (n=17) with high transduction efficiency and viability. Using this approach, we now have the opportunity to determine whether CD38 can directly alter gene expression in primary CLL cells and influence cell survival, migration and proliferation.
Design and Methods
Lymphocyte separation
Following informed consent, peripheral blood samples from CLL patients with low expression of CD38 were separated using Ficoll-Hypaque (Sigma, Poole, UK), washed in PBS and counted. Patients were diagnosed using morphological and immunophenotyping criteria and were treatment free for at least three months prior to their analysis.
Generation of lentivirus
A cDNA corresponding to CD38 (Accession NM_001775) or the first 233 amino acids of rat CD29 was cloned into the pHR’ SINcPPT SFFV-WPRE vector. Transgene expression was under the control of the SFFV promoter.10 The GFP virus, driven by the same promoter, has been previously described.10 The vector plasmids (pLentiSEW, pLentiSCD38W or pLentiSrCD2ΔW), together with the gag-pol plasmid (pΔ8.91) and the VSVG envelope encoding plasmid (pMD2-G), were amplified in bacteria and purified with the Endofree Maxiprep Kit (Qiagen). The transfer vector (13μg), pΔ8.91 (10μg) and pMD2-G (6 μg) was mixed with 1.5 mL of CaCl (0.25M) (Sigma Poole, UK). This was added to 1.5 mL of 2X HEPES (Sigma Poole, UK) while bubbling. The solution was left for 20 min to allow a precipitate to form. This was then added to a large flask (175 cm2) of 293T cells (approximately 60% confluent) containing 20 mL of Dulbecco’s Modified Essential Media (DMEM) with 10% fetal calf serum, 100 units/mL penicillin, 100 μg/mL streptomycin and 2 mM glutamine.11 After 48h, at 37°C, in 5% CO2, the supernatant was removed and centrifuged at 1,700 g for 10 min to pellet any cell debris, followed by ultracentrifugation at 121,603 g for two hours to concentrate the virus. The pellet, containing concentrated virus, was re-suspended in DMEM, (Invitrogen, Paisley, UK) without supplements and stored at −80°C.
Lentiviral infection of chronic lymphocytic leukemia cells
Primary CLL cells were added to DMEM cell culture media with supplements (10% FCS, 100 units/mL penicillin, 100 μg/mL streptomycin, 2 mM glutamine). Concentrated viral supernatant was added to the culture media. The cells were then incubated at 37°C, in 5% CO2. Expression of CD38 and other molecules were typically monitored after 48 h. No feeder cells or cytokines were added to the cultures.
Titration of lentivirus
The lentivirus was titrated using CLL cells. Five hundred thousand CLL cells were placed in 1 mL of DMEM cell culture media with 10% FCS, 100 units/mL penicillin, 100 μg/mL streptomycin, 2mM glutamine. Volumes of viral supernatant, ranging from 1μL to 128 μL, were added. After 48 h, gene expression was monitored and the number of infectious virus particles per microliter was estimated by determining the percentage of cells infected in the linear portion of the curve. In some cases, the amount of the lentiviral protein, p24, was determined by ELISA (Helvetica Health Care Sàrl, Switzerland).
Flow cytometry
The following antibodies were used for immunophenotypic analysis: anti-CD19PE-Cy5 from DACO (C7066), anti-CD38RPE from Caltag (MHCD 3804-4) and anti-CD2FITC from Santa Cruz Biotechnology (sc-53036). Expression was measured using a Becton Dickinson FACSCalibur.12
Results and Discussion
Lentiviral technology represents a powerful method of genetically modifying quiescent cells.13 Three decisions underpinned the development of this protocol. Firstly, we chose to use a viral backbone where transgene expression was driven by the spleen focus forming virus promoter. The CMV promoter has been shown to be ineffective in some quiescent lymphocytes.14 Secondly, we focused on CD38 as a candidate molecule that is important for CLL prognosis.15 Finally, CD38 is a cell surface marker, detected using flow cytometry, so the effectiveness of the genetic modification could be easily monitored. The virus generated to express CD38 was compared to two other viruses: a virus containing the genetic material for GFP and a virus containing the genetic material for truncated rat CD2.
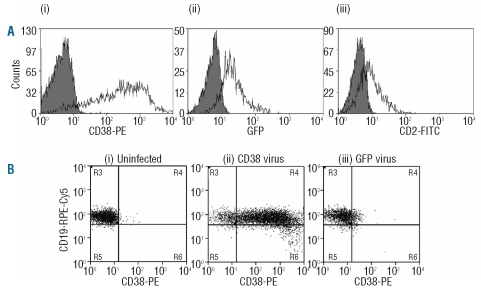
All three viruses were capable of expressing their transgene in primary human CLL cells following lentiviral infection (Figure 1A). The highest level of expression was detected for CD38. We investigated CD38 expression in the CD19 positive population infected with both the CD38 virus and the GFP virus. A dramatic increase in CD38 was observed following infection with the CD38 virus (Figure 1B). This contrasted with a small increase in CD38 expression following infection with the GFP-expressing virus (Figure 1B).
Figure 1.
Successful transduction of CLL cells with lentivirus. (A) Treatment of CLL cells with lentivirus containing the genetic codes for CD38 (i), GFP (ii), and a truncated rat CD2 (iii) resulted in high levels of transduction (87%, 70% and 43%, respectively). (B) CLL cells from a CD38 negative patient (i) were infected with a CD38 lentivirus and 94% transduction was achieved (ii). Infection with a GFP control lentivirus saw a 9.7% increase in CD38 expression (iii).
Figure 2A and B show CD38 expression following increasing multiplicity of infections (MOI) of both the CD38 and the GFP lentiviruses. A dose response was observed depending on the amount of CD38 virus used. This dose response was apparent for both the percentage of cells that were CD38 positive (Figure 2A) and the mean fluorescent intensity for CD38 expression (Figure 2B). It was, therefore, possible to select a dose of lentivirus which would allow the expression of physiological levels of CD38 on the surface of the CLL cells. Importantly, the expression from the CD38 virus at an MOI of one, was higher than the expression of CD38 seen following infection with the GFP virus, even with an MOI above ten. The number of viral particles, determined by ELISA, in both preparations was comparable. CD38 mRNA expression, measured by quantitative PCR, was higher in CD38 virus treated samples than in untreated or GFP virus treated samples (Online Supplementary Figure S1). The expression of CD38 was sustained over five days (Figure 2C and D).
Figure 2.
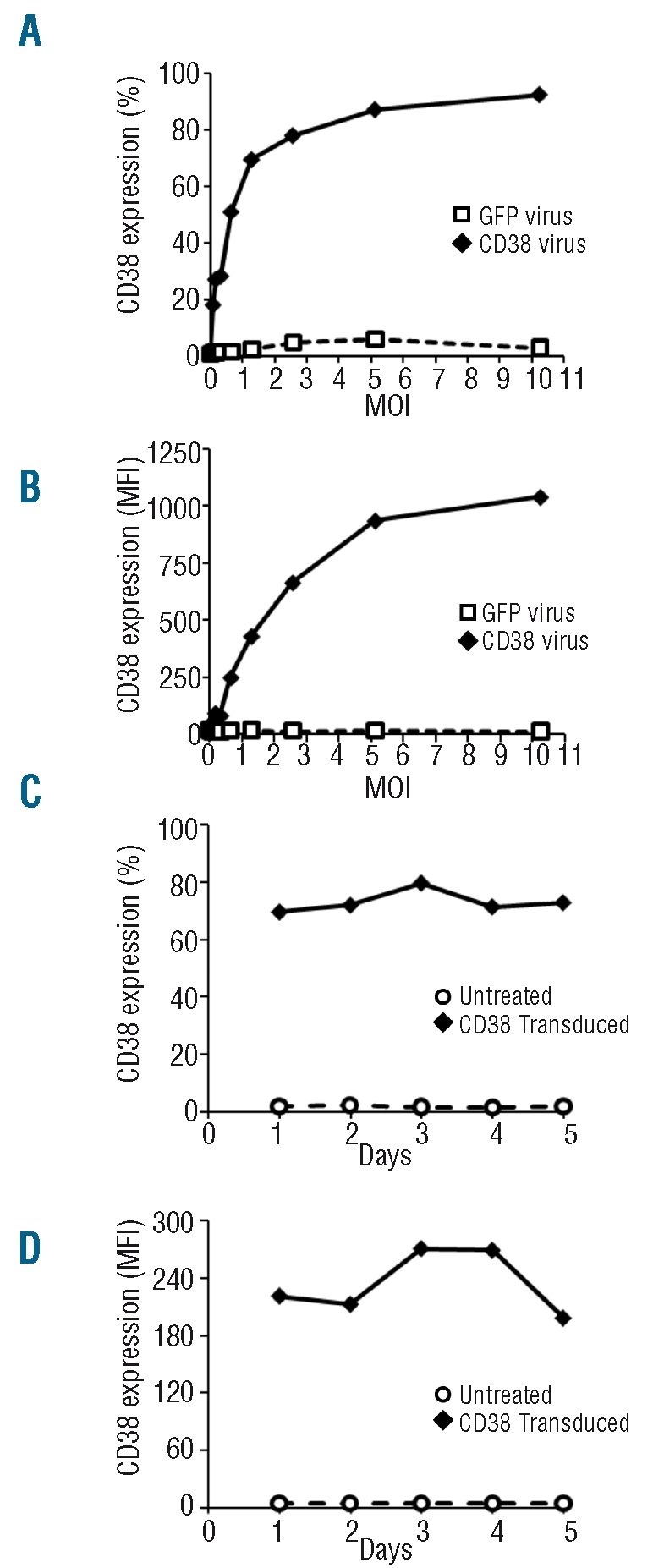
Expression of CD38 on virally infected CLL cells. A dose dependent increase in CD38 expression was observed in both the number of cells expressing CD38 (A) and in the MFI (B) of samples following treatment with CD38 virus (diamonds). No such increase is observed following the addition of a comparable amount of GFP virus (squares). Following transduction a high level of CD38 expression was observed on the surface of CLL cells following 24 h incubation. This level was sustained over a period of five days as measured by the percentage of cells expressing the CD38 antigen (C) and by the MFI (D) of the sample.
Given the heterogeneity of CLL, we investigated the changes in CD38 expression following viral infection in multiple patient samples. A high percentage of cells expressing CD38 was observed in all patient samples treated with the CD38 lentivirus (Figure 3A; n=17, mean percentage of positive cells (±SD) was 87% ±8.5%). To investigate the effect of lentivirus on cell differentiation, we analyzed the cells following treatment with lentivirus and observed no major changes in CLL cell morphology (Figure 3B), and only observed a small increase in the expression of CD138, which is highly expressed in antibody-secreting plasmacytoid cells (data not shown). Thus, we demonstrated reproducible transduction of primary CLL cells to manipulate the expression of CD38 without causing cell differentiation. Here, we describe a lentiviral technique which is able to transduce CLL cells to a level greater than previously described. In two studies, no GFP was detected when driven by a CMV promoter16,17 using VSVG coated lentivirus, which suggests that our use of the SFFV promoter allows higher levels of expression in CLL cells. Given the importance of promoters, using B-cell specific18 or other gene expression units19 may be useful. A report, published since submission, has demonstrated gene expression in CLL cells using lentivirus incorporating measles virus glycoproteins, H and F, on their surface.16 This allowed genetic modification of a subset of cells (20–45% of cells). In contrast, our data shows a change in the whole population of CLL cells rather than a subset and a technique for CD38 which allows genetic modification of an average of 87% of cells. This high level of expression means there is no need for cell sorting for further studies. Our data also shows that CD38 can be detected at a higher level than GFP (despite the amount of virus used, MFI of GFP infected cells is shown in the Online Supplementary Figure 2) or CD2, which may be due to the human origin of CD38.
Figure 3.
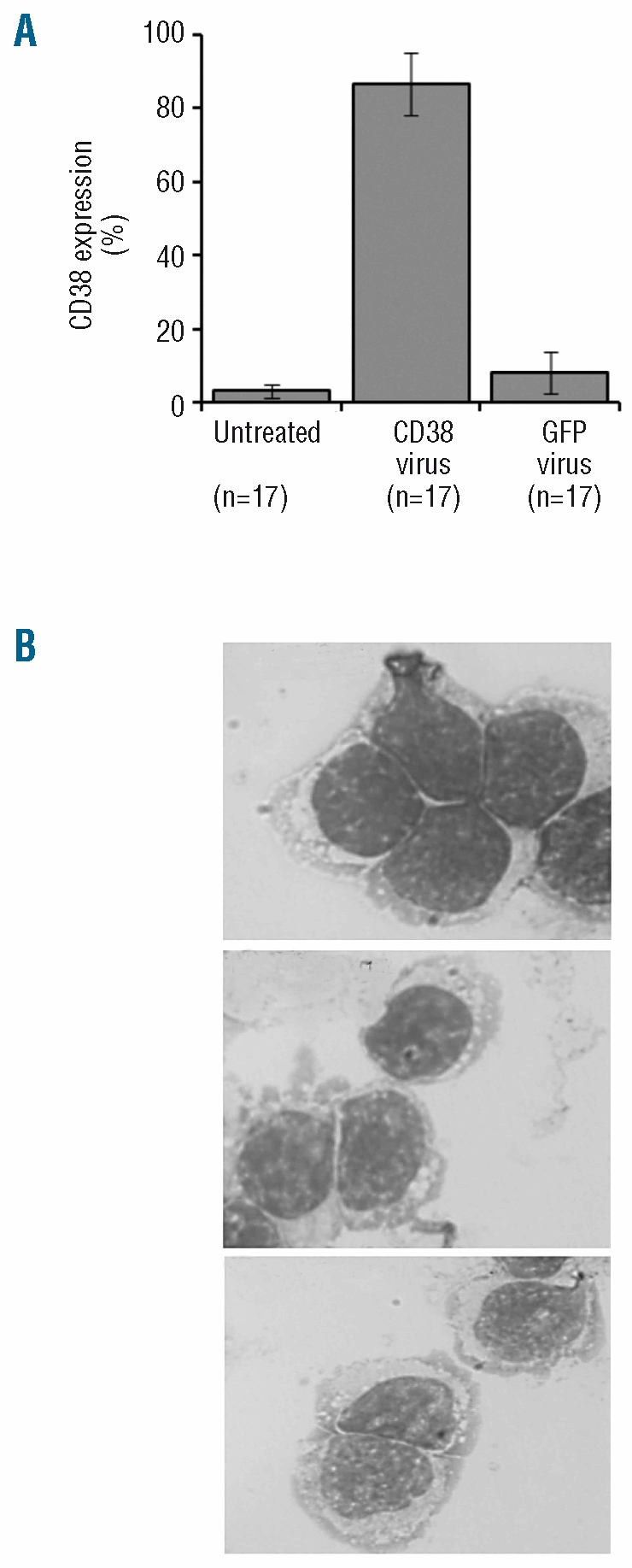
Expression of CD38 in multiple patient samples. (A) The mean expression of CD38 in the untreated samples was 3±1.8% (n=17). Following treatment with CD38 virus a mean of 87±8.5% (n=17) of CLL cells expressed the CD38 antigen. A mean of 8±5.8% CD38 expression (n=7) was observed in samples treated with control GFP virus. (B) Morphology of untreated and lentivirus treated CLL cells following 48 h incubation. Pictures were taken using a Zeiss Axio microscope equipped with a digital camera following Giemsa staining (×100 magnification).
The next step in our study is to characterize the functional effects of CD38 in CLL cells. Our initial experiments indicate that CD38 has a subtle effect on the survival of CD38 negative cells when expressed alone from the lentivirus. Our analysis of gene expression shows that CD38 is highly up-regulated at the mRNA level (data not shown) but analysis of expression patterns is confounded by the heterogeneous nature of CLL patient samples. Our immunophenotypic analysis shows that CD19 expression is not altered and the levels of CD138 (data not shown) do not indicate differentiation of the CLL cells. However, an analysis of other cell surface markers and of the effects of ligating CD38 are currently underway.
In conclusion, this report describes the successful genetic modification of primary CLL cells to generate a CD38 positive population from a CD38 negative population. This method has been successful on all the samples we have tested to date and does not require any other treatment of the cells.
Acknowledgments
The authors would like to thank Dr. Qasim and Professor Thrasher at the Institute of Child Health, London for the provision of reagents.
Footnotes
The online version of this article has a supplementary appendix.
Authorship and Disclosures
LP performed research, analyzed data and wrote paper; LM, TTL, SH and RJM performed research; SD and CR contributed vital reagents; CF contributed vital new reagents and revised the manuscript; CP and PB designed and performed research, analyzed data and wrote the paper.
The authors reported no potential conflicts of interest.
Funding: this work was supported by grants from the Leukaemia Research Appeal for Wales and Leukaemia Research UK.
References
- 1.Damle RN, Wasil T, Fais F, Ghiotto F, Valetto A, Allen SL, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94(6):1840–7. [PubMed] [Google Scholar]
- 2.Morabito F, Mangiola M, Oliva B, Stelitano C, Callea V, Deaglio S, et al. Peripheral blood CD38 expression predicts survival in B-cell chronic lymphocytic leukemia. Leuk Res. 2001;25(11):927–32. doi: 10.1016/s0145-2126(01)00049-2. [DOI] [PubMed] [Google Scholar]
- 3.Morabito F, Damle RN, Deaglio S, Keating M, Ferrarini M, Chiorazzi N. The CD38 ectoenzyme family: advances in basic science and clinical practice. Mol Med. 2006;12(11–12):342–4. doi: 10.2119/2006-00110.Morabito. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durig J, Naschar M, Schmucker U, Renzing-Kohler K, Holter T, Huttmann A, et al. CD38 expression is an important prognostic marker in chronic lymphocytic leukaemia. Leukemia. 2002;16(1):30–5. doi: 10.1038/sj.leu.2402339. [DOI] [PubMed] [Google Scholar]
- 5.Del Poeta G, Maurillo L, Venditti A, Buccisano F, Epiceno AM, Capelli G, et al. Clinical significance of CD38 expression in chronic lymphocytic leukemia. Blood. 2001;98(9):2633–9. doi: 10.1182/blood.v98.9.2633. [DOI] [PubMed] [Google Scholar]
- 6.Ibrahim S, Keating M, Do KA, O’Brien S, Huh YO, Jilani I, et al. CD38 expression as an important prognostic factor in B-cell chronic lymphocytic leukemia. Blood. 2001;98(1):181–6. doi: 10.1182/blood.v98.1.181. [DOI] [PubMed] [Google Scholar]
- 7.Pepper C, Ward R, Lin TT, Brennan P, Starczynski J, Musson M, et al. Highly purified CD38+ and CD38- sub-clones derived from the same chronic lymphocytic leukemia patient have distinct gene expression signatures despite their monoclonal origin. Leukemia. 2007;21(4):687–96. doi: 10.1038/sj.leu.2404587. [DOI] [PubMed] [Google Scholar]
- 8.McCabe D, Bacon L, O’Regan K, Condron C, O’Donnell JR, Murphy PT. CD38 expression on B-cell chronic lymphocytic leukemic cells is strongly correlated with vascular endothelial growth factor expression. Leukemia. 2004;18(3):649–50. doi: 10.1038/sj.leu.2403282. [DOI] [PubMed] [Google Scholar]
- 9.He Q, Beyers AD, Barclay AN, Williams AF. A role in transmembrane signaling for the cytoplasmic domain of the CD2 T lymphocyte surface antigen. Cell. 1988;54(7):979–84. doi: 10.1016/0092-8674(88)90112-2. [DOI] [PubMed] [Google Scholar]
- 10.Demaison C, Parsley K, Brouns G, Scherr M, Battmer K, Kinnon C, et al. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency [correction of imunodeficiency] virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum Gene Ther. 2002;13(7):803–13. doi: 10.1089/10430340252898984. [DOI] [PubMed] [Google Scholar]
- 11.Qasim W, Mackey T, Sinclair J, Chatziandreou I, Kinnon C, Thrasher AJ, et al. Lentiviral vectors for T-cell suicide gene therapy: preservation of T-cell effector function after cytokine-mediated transduction. Mol Ther. 2007;15(2):355–60. doi: 10.1038/sj.mt.6300042. [DOI] [PubMed] [Google Scholar]
- 12.Pepper C, Lin TT, Pratt G, Hewamana S, Brennan P, Hiller L, et al. Mcl-1 expression has in vitro and in vivo significance in chronic lymphocytic leukemia and is associated with other poor prognostic markers. Blood. 2008;112(9):3807–17. doi: 10.1182/blood-2008-05-157131. [DOI] [PubMed] [Google Scholar]
- 13.Qasim W, Gaspar HB, Thrasher AJ. Gene therapy for severe combined immune deficiency. Expert Rev Mol Med. 2004;6(13):1–15. doi: 10.1017/S1462399404007884. [DOI] [PubMed] [Google Scholar]
- 14.Hurez V, Dzialo-Hatton R, Oliver J, Matthews RJ, Weaver CT. Efficient adenovirus-mediated gene transfer into primary T cells and thymocytes in a new coxsackie/adenovirus receptor transgenic model. BMC Immunol. 2002;3:4. doi: 10.1186/1471-2172-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deaglio S, Aydin S, Vaisitti T, Bergui L, Malavasi F. CD38 at the junction between prognostic marker and therapeutic target. Trends Mol Med. 2008;14(5):210–8. doi: 10.1016/j.molmed.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Frecha C, Costa C, Levy C, Negre D, Russell SJ, Maisner A, et al. Efficient and stable transduction of resting B-lymphocytes and primary chronic lymphocyte leukemia cells using measles virus gp displaying lentiviral vectors. Blood. 2009;114(15):3173–80. doi: 10.1182/blood-2009-05-220798. [DOI] [PubMed] [Google Scholar]
- 17.Van Bockstaele F, Pede V, Naessens E, Van Coppernolle S, Van Tendeloo V, Verhasselt B, et al. Efficient gene transfer in CLL by mRNA electroporation. Leukemia. 2008;22(2):323–9. doi: 10.1038/sj.leu.2405007. [DOI] [PubMed] [Google Scholar]
- 18.Laurie KL, Blundell MP, Baxendale HE, Howe SJ, Sinclair J, Qasim W, et al. Cell-specific and efficient expression in mouse and human B cells by a novel hybrid immunoglobulin promoter in a lentiviral vector. Gene Ther. 2007;14(23):1623–31. doi: 10.1038/sj.gt.3303021. [DOI] [PubMed] [Google Scholar]
- 19.Zhang F, Thornhill SI, Howe SJ, Ulaganathan M, Schambach A, Sinclair J, et al. Lentiviral vectors containing an enhancer-less ubiquitously acting chromatin opening element (UCOE) provide highly reproducible and stable transgene expression in hematopoietic cells. Blood. 2007;110(5):1448–57. doi: 10.1182/blood-2006-12-060814. [DOI] [PMC free article] [PubMed] [Google Scholar]