Abstract
Retinitis pigmentosa (RP) is a collection of diseases in which rod photoreceptors die from a variety of mutations. After rods die, the level of tissue oxygen in the outer retina becomes elevated and there is progressive oxidative damage to cones that ultimately triggers apoptosis. In this study, we investigated the hypothesis that NADPH oxidase and/or xanthine oxidase serve as critical intermediaries between increased tissue oxygen and the generation of excessive reactive oxygen species that cause oxidative damage to cones. Apocynin, a blocker of NADPH oxidase, but not allopurinol, a blocker of xanthine oxidase, markedly reduced the superoxide radicals visualized by hydroethidine in the outer retina in the retinal degeneration-1 (rd1+/+) model of RP. Compared to rd1+/+ mice treated with vehicle, those treated with apocynin, but not those treated with allopurinol, had significantly less oxidative damage in the retina measured by ELISA for carbonyl adducts. Apocynin-treated, but not allopurinol-treated, rd1+/+ mice had preservation of cone cell density, increased mRNA levels for m- and s-cone opsin, and increased mean photopic b-wave amplitude. In Q344ter mice, a model of dominant RP in which mutant rhodopsin is expressed, apocynin treatment preserved photopic electroretinogram b-wave amplitude compared to vehicle-treated controls. These data indicate that NADPH oxidase, but not xanthine oxidase, plays a critical role in generation of the oxidative stress that leads to cone cell death in RP and inhibition of NADPH oxidase provides a new treatment strategy.
Keywords: antioxidants, apoptosis, photoreceptors, reactive oxygen species, retina, retinal dystrophies
Introduction
Retinitis pigmentosa (RP) is a group of diseases caused by a wide variety of mutations that compromise rod photoreceptors. Some of the mutations are in genes that are specifically expressed in rods, such as rhodopsin (Dryja et al. 1990) or rod phosphodiesterase (McLaughlin et al. 1993). Others are in genes that affect cilia (Koenig 2003). Since cilia are a critical component of rods and the hair cells of the inner ear, those mutations often lead to loss of vision and hearing. Some mutations are in genes that are ubiquitously expressed, but for some reason lead to selective death of rod photoreceptors (Daiger et al. 2007). Defining the mechanism by which these various mutations cause the death of rods is a major area of research, because one approach to treatment development is to find ways of countering these mechanisms of cell death to preserve rods (Sancho-Pelluz et al. 2008). Given the large number of mutations and the likelihood that many different mechanisms of rod cell death may be involved, and that many of them, such as protein misfolding, may be very difficult to reverse, this is a daunting task.
With loss of rods, patients become unable to see in dim illumination, but retain the ability to read and drive when lighting is sufficient. These activities are mediated by cone photoreceptors, which remain intact until most of the rods have died and then they begin to die. The gradual death of cones causes gradual constriction of visual fields leaving central islands that are eventually snuffed out. If cones could be preserved, patients would function very well despite loss of rods.
Recently, our laboratory has demonstrated that oxidative damage is a major contributing factor to cone cell death after the death of rods has occurred (Shen et al. 2005; Komeima et al. 2006; Komeima et al. 2007). Rods are the most numerous and most metabolically active cell type in the retina and thus the biggest consumers of oxygen. After rods die, the level of tissue oxygen in the outer retina becomes markedly elevated (Stone et al. 1999; Yu et al. 2000; Yu et al. 2004; Padnick-Silver et al. 2006). The increased levels of oxygen result in progressive oxidative damage to cones in a transgenic pig model of RP (Shen et al. 2005) and in multiple mouse models, including models of recessive and dominant RP (Komeima et al. 2006; Komeima et al. 2007) Administration of a cocktail of antioxidants to rd1+/+ mice reduces markers of oxidative damage in cones, reduces cone cell loss, and preserves the electrical function of cones, indicating that oxidative damage plays a central role in cone cell death regardless of the underlying mutation that kills rods.
An important unanswered question is how does elevation of tissue oxygen in the outer retina lead to progressive oxidative damage in cones? Under normal conditions, mitochondria are one source of reactive oxygen species (ROS), because the highly reducing environment in mitochondria makes it possible for molecules such as flavoproteins, iron-sulfur clusters, or ubisemiquinone to transfer one electron to oxygen and hence generate superoxide radicals (Turrens 2003). The rate of superoxide radical production by the electron transport chain is controlled by mass action and increases when electron flow slows down causing a relative increase in the number of electron donors or when the concentration of oxygen is increased (Turrens et al. 1982). In addition to this accidental generation of ROS which is magnified by hyperoxia, there are systems that deliberately produce ROS. One such system is the NADPH oxidase (Nox) system. The Nox of phagocytes (Phox) was the first to be characterized and is a critical component of the innate immunity system, because it generates ROS that help to kill invading microbes (Babior et al. 2002). It is a complex enzyme consisting of a catalytic subunit, gp91phox (also know as Nox2), the regulatory subunits p22phox, p47phox, p67 phox, and the small GTPase RAC (Lambeth et al. 2007). Subsequently homologues of Nox2 were identified (Nox1, 3, 4, and 5, and dual oxidases (Duox) 1 and 2 and were found to be expressed in a variety of non-phagocytic cells. Members of the Nox family of enzymes generate superoxide radicals by one electron-reduction of molecular oxygen by NADPH(Isogai et al. 1995). The p22phox subunit has been demonstrated to be present in whole retina by Western blot and by in situ hybridiazation, its mRNA has been identified in essentially all cells in the retina including photoreceptor inner segments, although constitutive levels are highest in ganglion cells and retinal pigmented epithelial cells(Li et al. 2008). Since oxygen is a substrate, hyperoxia should cause increased production of superoxide radicals by Nox enzymes, but whether this occurs in the outer retina is unknown.
The major function of xanthine oxidase is in the two terminal reactions of the purine degradation pathway, the conversion of hypoxanthine to xanthine and the conversion of xanthine to uric acid. (Hille and Nishino 1995) These reactions generate reduced xanthine oxidase and its reoxidation generates two superoxide and two H2O2 radicals. (Hille and Massey 1981) Levels of xanthine oxidase are highest in liver, but it is also present in capillary endothelial cells and heart. (Jarasch et al. 1981; Bruder et al. 1983; Linder et al. 1999) Its presence in endothelial cells means xanthine oxidase is present in all tissues and thus is a ubiquitous source of ROS. It has been implicated in ischemia-reperfusion injury, vascular diseases including atherosclerosis, and heart failure. (Chambers et al. 1985; Brown et al. 1988; Terada et al. 1992; Cappola et al. 2001; Patetsios et al. 2001) In this study, we tested the hypothesis that NADPH oxidase and/or xanthine oxidase contribute to oxidative damage and cone cell death in models of RP.
Materials and Methods
Treatment with inhihibitors of NADPH Oxidase or Xanthine Oxidase
Mice were treated in accordance with the recommendations of the Association for Research in Vision and Ophthalmology. Litters of homozygous rd1+/+ mice or heterozygous Q344ter transgenic mice were separated into two groups. Mice in one group were given daily intraperitoneal injection of 10 mg/kg of apocynin, a Nox inhibitor, or 100 mg/kg of allopurinol, a xanthine oxidase inhibitor. These doses were selected based upon previous studies that had demonstrated their effectiveness.(Augustin et al. 1994; Al-Shabrawey et al. 2005; Saito et al. 2007) All drugs were obtained from Sigma Aldrich (Saint Louis, MO). Apocynin was dissolved in warm phosphate-buffered saline (PBS) and allopurinol was dissolved in 1M NaOH in distilled water followed by titration to pH10 by 1M HCl. The control group for apocynin-treated mice was given injections of PBS and the control group for allopurinol-treated mice was given injections of pH10 vehicle.
Assessment of superoxide radicals with hydroethidine
The in situ production of superoxide radicals was evaluated using hydroethidine as previously described (Behrens et al. 2008; Komeima et al. 2008). In the presence of superoxide radicals, hydroethidine is converted to ethidium, which binds DNA and emits red fluorescence at approximately 600nm. Briefly, mice were given two 20 mg/kg intraperitoneal injections of freshly prepared hydroethidine (Invitrogen, Carlsbad, CA) 30 minutes apart and euthanized 18 hours after injection. Eyes were rapidly removed and 10 µm frozen sections were fixed in 4% paraformaldehyde for 20 minutes at room temperature, rinsed with PBS, and counterstained for 5 minutes at room temperature with the nuclear dye Hoechst 33258 (1:10000; Sigma, St. Louis, MO). After rinsing in PBS, slides were examined by fluorescence microscopy (excitation: 543nm, emission>590nm) with a Zeiss LSM 510 META confocal microscope using a Zeiss Plan-Apochromat 20x/0.75NA objective lens (Carl Zeiss, Oberkochen, Germany). All images were acquired in the frame scan mode with the same exposure time. The excitation wavelength was set at 405 nm for visualization of Hoechst.
ELISA for protein carbonyl content
Retinas were homogenized in lysis buffer and centrifuged at 16,000 × g for 5 minutes at 4°C and the protein concentration of the supernatant was measured using a Bio-Rad Protein Assay Kit (Bio-Rad, Hercules, CA). Samples were adjusted to 4 mg/ml by dilution with Tris-buffered saline, pH 7.4 (TBS), and protein carbonyl content was determined by ELISA as previously described (Komeima et al. 2006; Lu et al. 2008).
Measurement of cone cell density
Cone density was measured as previously described (Komeima et al. 2006). Briefly, each mouse was euthanized, and eyes were removed and fixed in 4% paraformaldehyde overnight at 4°C. After washing with PBS, the cornea, iris, and lens were removed. A small triangular cut was made at 12:00 for orientation and after 4 radial cuts equidistant around the circumference, the entire retina was carefully dissected from the eye cup and any adherent retinal pigmented epithelium (RPE) was removed. Retinas were placed in PBS containing 0.1% Tween 20 (Promega Corporation, Madison, WI) for 30 minutes at room temperature, incubated for 1 hour at room temperature in 1:100 rhodamine-conjugated peanut agglutinin (PNA; Vector Laboratories, Burlingame, CA) in PBS containing 0.1% Tween 20. The retinas were rinsed 3 times for 10 minutes in PBS containing 0.1% Tween 20, given a final rinse in PBS and flat-mounted. The retinas were examined with a Zeiss LSM 510 META confocal microscope with a Zeiss Plan-Apochromat 10×/0.17NA objective lens for low magnification or 20×/0.75NA objective lens for high magnification using an excitation wavelength of 543 nm to detect rhodamine fluorescence. Images were acquired in the frame scan mode. High magnification images for cone cells were taken within four 230 µm × 230 µm squares located 0.5 mm superior, inferior, temporal and nasal to the center of the optic nerve. The investigator was masked with respect to experimental group.
Measurement of mRNA levels in the retina by real-time RT-PCR
Starting at P11, rd1+/+ mice received daily intraperitoneal injections of vehicle or vehicle containing 10 mg/kg apocynin, or vehicle containing 100 mg/kg allopurinol. Mice were euthanized at P25 and eyes were removed. Retinas were dissected and total retinal RNA was isolated using RNeasy kits (QIAGEN Inc., Chatsworth, CA). After treatment with DNase I (Invitrogen, Calsbad, CA), 1 µg of RNA was incubated with SuperScript III reverse transcriptase (Invitrogen) and 5 µM oligo-d(T) primer (Invitrogen). Samples of cDNA were aliquoted and stored at −80 °C. Real-time polymerase chain reaction was performed using a Light Cycler rapid thermal cycler system (Roche Applied Bioscience). Cyclophilin A was used as a control for normalization. Primers specific for m-cone opsin (forward: 5’-TCA TTT CCT GGG AGA GAT GG-3’ and reverse: 5’-AGG CCA TAA GGC CAG TAC CT-3’), s-cone opsin (forward: 5’-GCC TCA GTA CCA CCT TGC TC-3’ and reverse: 5’-CTG GCG ATG AAG ACT GTG AA-3’), and cyclophilin A (forward: 5’-CAG ACG CCA CTG TCG CTT T-3’ and reverse: 5’-TGT CTT TGG AAC TTT GTC TGC AA-3’) were used. Standard curves generated with purified cDNA were used to calculate copy number according to the Roche absolute quantification technique manual.
Recording of electroretinograms (ERGs)
An Espion ERG Diagnosys machine (Diagnosys LLL, Littleton, MA) was used to record ERGs as previously described (Okoye et al. 2003; Komeima et al. 2006; Komeima et al. 2007; Ueno et al. 2008). The mice were anesthetized with an intraperitoneal injection of ketamine hydrochloride (100mg/kg body weight) and xylazine (5mg/kg body weight). Pupils were dilated with Midrin P containing of 0.5% tropicamide and 0.5% phenylephrine hydrochloride (Santen Pharmaceutical Co., Osaka, Japan). The mice were placed on a pad heated to 39°C and platinum loop electrodes were placed on each cornea after application of Gonioscopic prism solution (Alcon Labs, Fort Worth, TX). A reference electrode was placed subcutaneously in the anterior scalp between the eyes and a ground electrode was inserted into the tail. The head of the mouse was held in a standardized position in a ganzfeld bowl illuminator that ensured equal illumination of the eyes. Recordings for both eyes were made simultaneously with electrical impedance balanced. Low background photopic ERGs were recorded at 1.48 log cd-s/m2 under a background of 10 cd/m2. Sixty photopic measurements were taken and the average value was recorded.
Statistical analysis
Statistical comparisons were done using unpaired Student’s t-test or Welch’s t-test for two comparisons.
Results
Nox enzymes contribute to accumulation of superoxide radicals in the retinas of rd1+/+ mice
Rd1+/+ mice are homozygous for a nonsense mutation in exon 7 of the Pde6b gene, which completely disrupts production of the β subunit of rod phosphodiesterase (Carter-Dawson et al. 1978). The rods develop, but then undergo rapid degeneration between postnatal day (P) 12 and P21. Mutations in the Pde6b gene also cause RP in humans (McLaughlin et al. 1993). Therefore, rd1+/+ mice provide a model of rapidly progressive recessive RP.
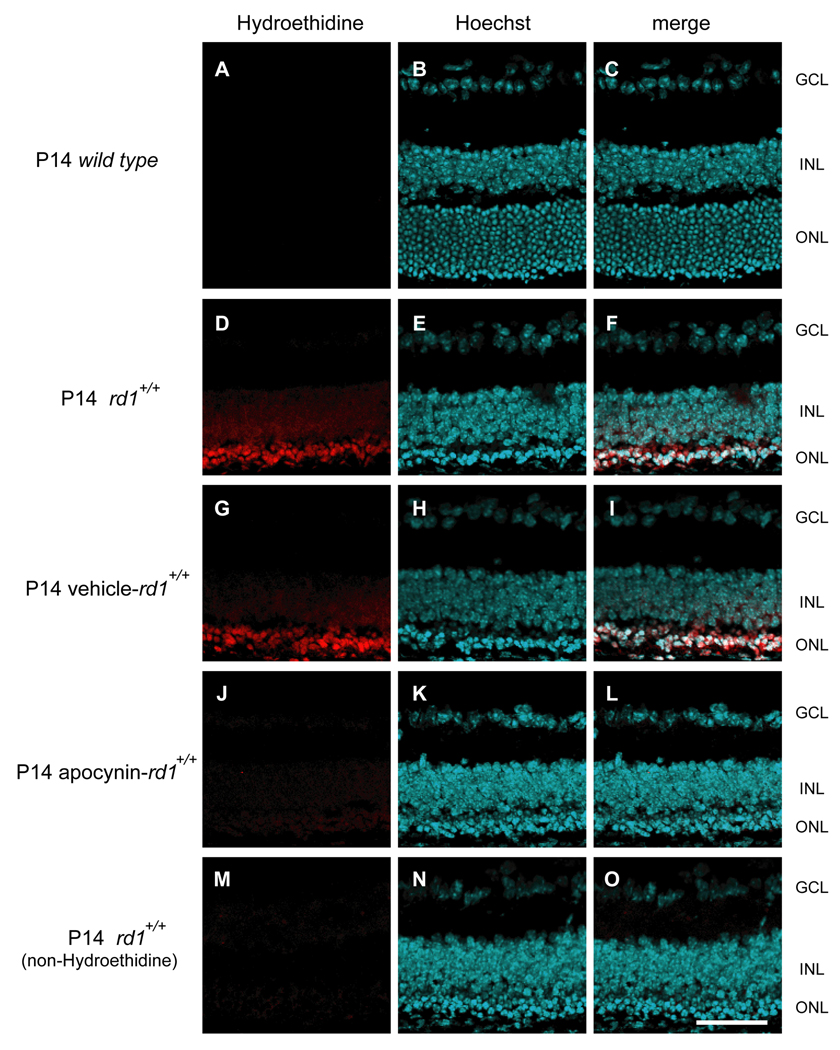
Injection of hydroethidine provides a sensitive indicator of superoxide radicals because in their presence it is converted to ethidium which binds DNA and fluoresces. At P14, wild type mice showed no fluorescence in the retina 18 hours after injection of hydroethidine (Figure 1A–C). At P14, many of the rods in the retinas of rd1+/+ mice have already degenerated and there was strong fluorescence in the outer retina after injection of hydroethidine (Figure 1D–F). Compared to rd1+/+ mice treated with vehicle which had prominent fluorescence in the outer retina (Figure 1G–I) like that seen in untreated rd1+/+ mice, those treated with 10 mg/kg of apocynin had much less fluorescence (Figure 1J–L) similar to that seen in rd1+/+ mice that were not injected with hydroethidine (Figure 1M–O). This demonstrates that one or more Nox enzyme contributes to the accumulation of superoxide radicals that occurs in the outer retina of rd1+/+ mice as rods degenerate.
Figure 1. Apocynin, an inhibitor of NADPH oxidase, reduces superoxide radicals in the retinas of rd1+/+ mice.
Rd1+/+ mice were given daily intraperitoneal injections of vehicle or vehicle containing 10mg/kg apocynin between postnatal day (P) 11 and P14. At P14, wild type mice, untreated rd1 mice or those treated with vehicle or apocynin (n=4 for each) were given two intraperitoneal injections of 20 mg/kg hydroethidine thirty minutes apart and after 18 hours they were euthanized and ocular sections were examined by confocal microscopy as described in Methods. There was minimal fluorescence in the retinas of wild type mice (A–C), strong fluorescence primarily in the remaining outer nuclear layer of the retinas of rd1 mice (D–F) and vehicle treated rd1+/+ mice (G–I), and minimal fluorescence in the retinas of apocynin treated rd1+/+ mice (J–L) or rd1+/+ mice that were not injected with hydroethidine (M–O). This demonstrates that NADPH contributes to the accumulation of superoxide radicals that occurs in the outer retina of rd1+/+ mice after rods have degenerated. Scale bar=50 µm
Nox enzymes contribute to oxidative damage in the retinas of rd1+/+ mice
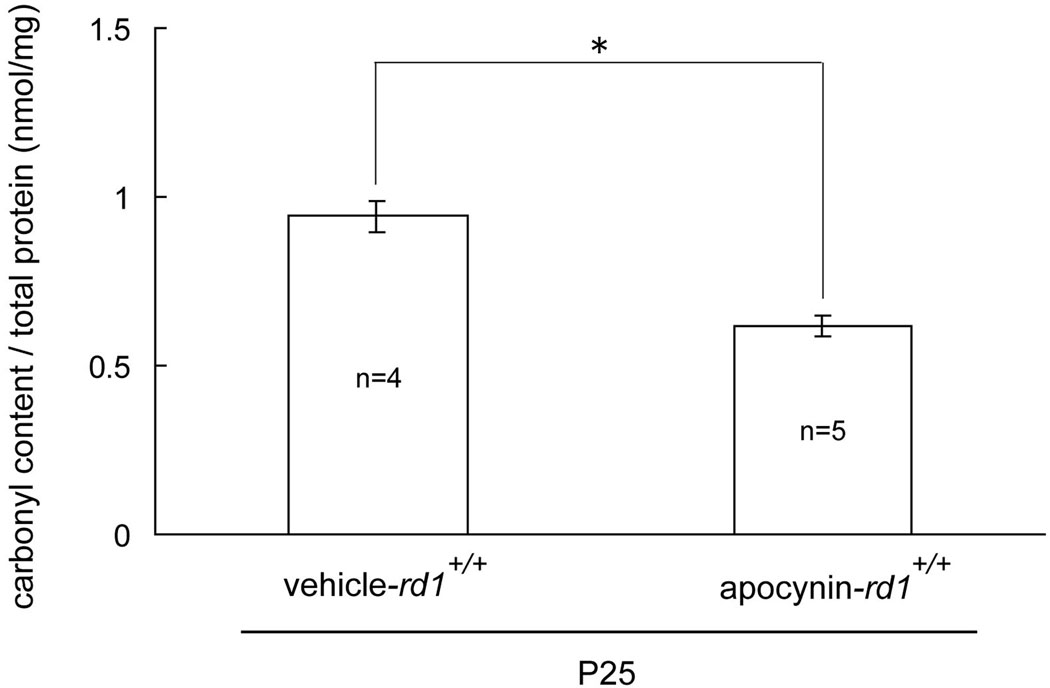
To determine if the reduction of superoxide radicals in apocynin-treated rd1+/+ mice resulted in reduced oxidative damage, we used ELISAs for carbonyl adducts on proteins, which provide a quantitative assessment. The carbonyl content per mg protein in the retina was significantly less in apocynin-treated P25 rd1+/+ mice compared to those treated with vehicle (Figure 2).
Figure 2. Apocynin significantly reduces carbonyl content in the retinas of postnatal day (P) 25 rd1+/+ mice.
Starting at P11, rd1+/+ mice were given daily intraperitoneal injections of vehicle or vehicle containing apocynin. Mice were euthanized at P25 and protein carbonyl content was measured by ELISA of retinal homogenates as described in Methods. The mean (±SEM) carbonyl content per mg retinal protein was significantly greater in vehicle-treated rd1+/+ mice compared to those treated with apocynin (*p<0.001 by unpaired Student’s t-test).
Blocking Nox promotes cone survival and function in the retinas of rd1+/+ mice
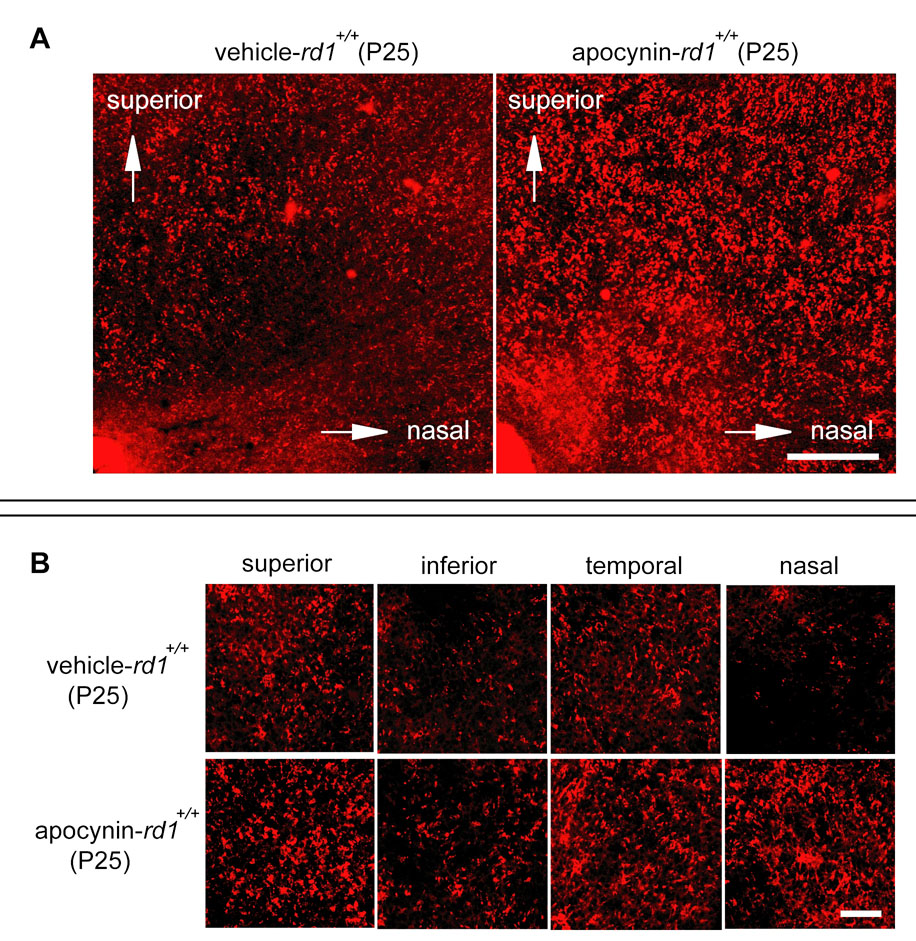
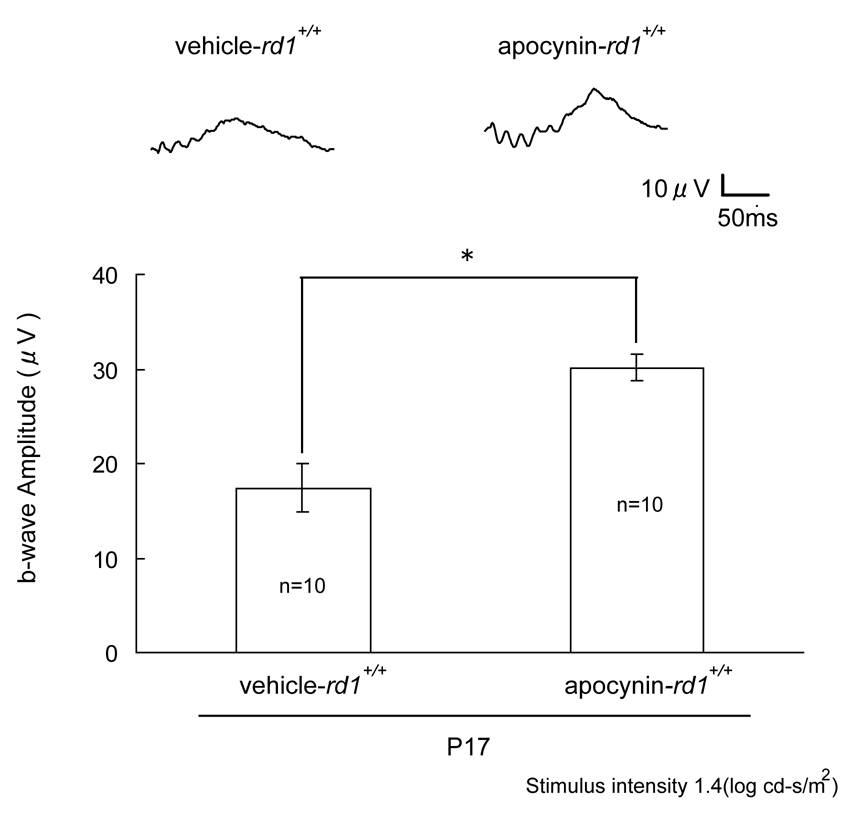
Rd1+/+ mice were treated with daily injections of vehicle or vehicle containing 10mg/kg apocynin between P11 and P25 and then euthanized. Whole mounts of retinas stained with rhodamine-labeled PNA, which selectively stains the matrix sheath around cone inner and outer segments, showed that compared to mice treated with vehicle, those treated with apocynin had greater cone density that was particularly dramatic in the superior nasal quadrant of the retina (Figure 3A), but was readily discernible in all four quadrants (Figure 3B). The level of mRNA for m-cone and s-cone opsin was measured by real time PCR and was significantly greater in apocynin-treated rd1+/+ mice compared to vehicle treated rd1+/+ mice (Figure 3C). This provides another objective measure of cone survival, but also indicates that an important function, transcription, was maintained by blocking Nox. Photopic ERGs provide a more direct measure of cone function, and compared to vehicle-treated rd1+/+ mice, those treated with apocynin had significantly greater photopic b-wave amplitude at P25 (Figure 4). Thus, blockade of Nox maintained cone viability and function in rd1+/+ mice.
Figure 3. Apocynin promotes cone survival in rd1+/+ mice.
Starting at P11, rd1+/+ mice were given daily intraperitoneal injections of vehicle or vehicle containing apocynin. Mice were euthanized at P25 and retinas were used for real time RT-PCR or stained with peanut agglutinin (PNA) and whole mounted as described in Methods.
(A) Low magnification images of the superonasal quadrant of the retina show a higher density of cones in apocynin-treated rd1+/+ mice compared with vehicle treated rd1+/+ mice. The optic nerve is in the lower left hand corner of the images. Scale bar 200µm.
(B) High magnification images of 0.0529 mm2 bins 0.5 mm superior, inferior, temporal, and nasal to the optic nerve show a higher density of cones in all 4 retinal quadrants of apocynin-treated rd1+/+ mice compared to vehicle-treated rd1 mice. Scale bar 50µm.
(C) The level or mRNA for m-cone and s-cone opsins was significantly greater in apocynin-treated rd1+/+ mice compared to vehicle treated rd1+/+ mice (*p<0.05 by unpaired Welch’s t-test for m-cone opsin, **p<0.001 by unpaired Student’s t-test for s-cone opsin).
Figure 4. Apocynin promotes cone cell function in rd1+/+ mice.
Starting at P11, rd1+/+ mice were given daily intraperitoneal injections of vehicle or vehicle containing 10 mg/kg apocynin. Low background photopic ERGs were done as described in Methods at P17. Representative waveforms are shown for each group and illustrate a substantially better waveform for apocynin-treated rd1+/+ mice compared to vehicle-treated rd1+/+ mice. The bars show mean (± SEM) photopic b-wave amplitude, which was significantly higher for apocynin-treated rd1+/+ mice compared to vehicle treated rd1+/+ mice (*p<5.0×10−4 by unpaired Student’s t-test).
Blockade of Nox preserves cone function in Q344ter transgenic mice
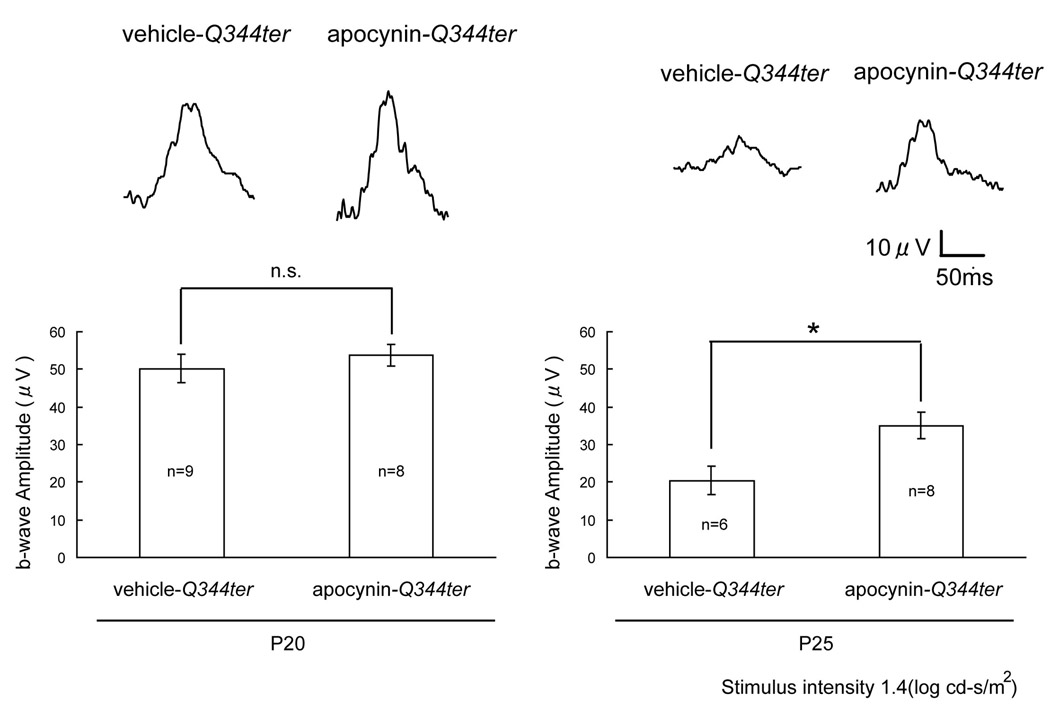
Transgenic mice that express truncated rhodopsin due to a mutation resulting in a stop codon at position 344, referred to as Q344ter mice, develop degeneration of rod photoreceptors starting about P10 and completed by about P21 (Sung et al. 1994). This same mutation has been identified in patients with dominant RP (Sung et al. 1991). Therefore, Q344ter mice provide a model of rapidly progressive dominant RP. Starting at P11, Q344ter transgenic mice were given daily intraperitoneal injections of vehicle or vehicle containing 10 mg/kg of apocynin. Low background photopic ERGs were done at P20 and P25. Photopic b-waves were quite good in both groups at P20 (Figure 5, left panel), but at P25, apocynin-treated mice had significantly higher photopic b-wave amplitude than vehicle-treated mice (Figure 5, right panel). This indicates that in a second model of RP, this one a model of dominant RP, blockade of Nox slows the loss of cone cell function.
Figure 5. Apocynin promotes cone cell function in Q344ter transgenic mice.
Starting at P11, Q344ter transgenic mice were given daily intraperitoneal injections of vehicle or vehicle containing 10 mg/kg apocynin. Low background photopic ERGs were done at P20 and P25. Representative waveforms are shown for each group and illustrate a substantially better waveform in apocynin-treated Q344ter transgenic mice compared to vehicle-treated Q344ter mice at P25. The bars show mean (± SEM) photopic b-wave amplitude, which showed no significant difference between apocynin-treated and vehicle-treated Q344ter transgenic mice at P20 (P=0.49 by unpaired Student’s t-test), but a significantly higher b-wave amplitude apocynin-treated mice at P25 (*p<0.05 by unpaired Welch’s t-test).
Blockade of xanthine oxidase has no effect on cone survival or function in rd1+/+ mice
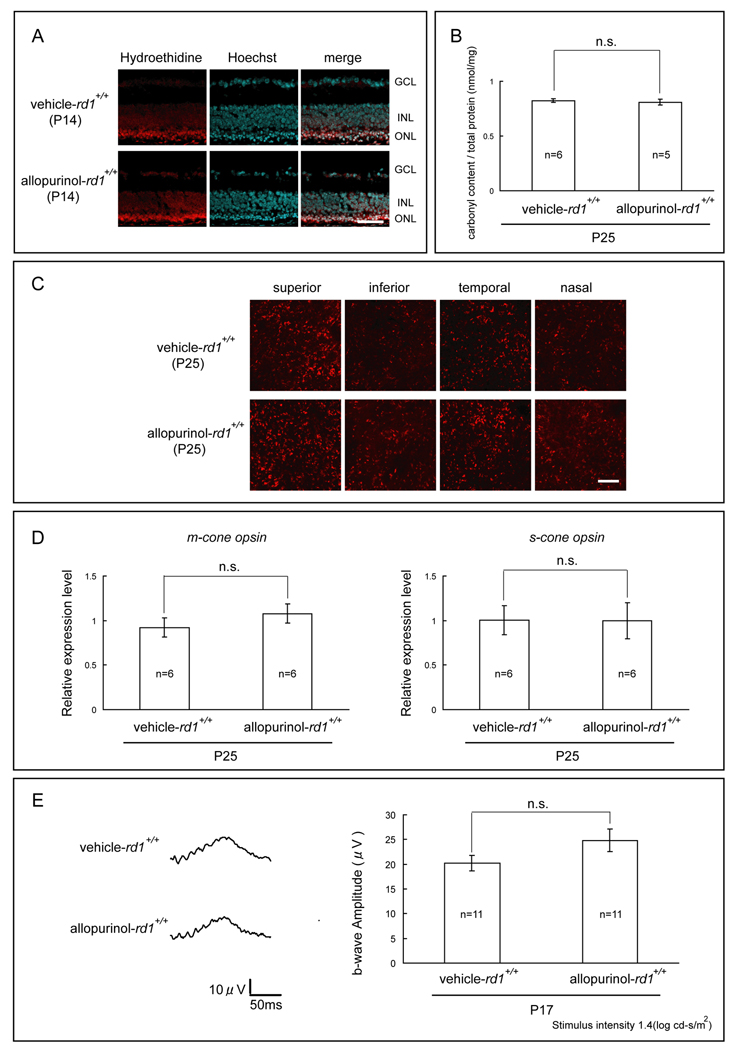
Xanthine oxidase, like Nox, is an endogenous generator of reactive oxygen species. To determine if xanthine oxidase contributes to oxidative damage in the retinas of rd1+/+ mice, we used allopurinol, which blocks xanthine oxidase. Starting at P11, rd1+/+ mice were given daily intraperitoneal injections of vehicle or vehicle containing 100mg/kg allopurinol. At P14, both vehicle-treated and allopurinol-treated rd1+/+ mice showed strong fluorescence in the remaining outer nuclear layer of the retina 18 hours after intraperitoneal injection of hydroethidine (Figure 6A), indicating that there was a substantial amount of superoxide radicals present in both. At P25, protein carbonyl content measured by ELISA was not significantly different in the retinas of allopurinol-treated rd1+/+ mice compared to vehicle-treated rd1+/+ mice (Figure 6B). Fluorescent confocal images of peanut agglutinin (PNA)-stained retinal flat mounts showed similar appearances of cone density in all 4 quadrants of the retina (Figure 6C), and the relative expression level of m-cone and s-cone opsin mRNA per retina was not significantly different (Figure 6D). At P17, photopic ERG b-wave amplitudes were equally reduced in both groups (Figure 6E).
Figure 6. The xanthine oxidase Inhibitor, allopurinol, does not reduce cone cell death in rd1+/+ mice.
Starting at P11, rd1+/+ mice in a C57BL/6 background were given daily intraperitoneal injections of vehicle or vehicle containing 100 mg/kg allopurinol.
(A) At P14, vehicle-treated and allopurinol treated rd1+/+ mice both showed strong fluorescence in the remaining outer nuclear layer of the retina 18 hours after intraperitoneal injection of hydroethidine. Scale bar=50 µm.
(B) At P25, protein carbonyl content measured by ELISA was not significantly different in the retinas of allopurinol-treated rd1+/+ mice compared to vehicle- treated rd1+/+ mice (P=0.65 by unpaired Student’s t-test).
(C) At P25, fluorescence confocal images of peanut agglutinin (PNA)-stained retinal flat mounts showed no difference in cone cell density in 0.0529 mm2 bins 0.5 mm superior, inferior, temporal and nasal to the center of the optic nerve for allopurinol-treated compared to vehicle-treated rd1+/+ mice. Scale bar=50 µm.
(D) At P25, the relative expression level of m-cone and s-cone opsin mRNA per retina was not significantly different in allopurinol-treated versus vehicle-treated rd1+/+ mice (P=0.33 by unpaired Welch’s t-test for m-cone, P=0.98 by unpaired Student’s t-test for s-cone).
(E) At P17, waveforms of low background photopic ERGs appeared the same for allopurinol- and vehicle-treated rd1+/+ mice and there was no significant difference in mean (± SEM) b-wave amplitude (P= 0.12 by unpaired Welch’s t-test).
Discussion
In RP, once rods die from the pathogenic mutation, cones undergo progressive oxidative damage and scavenging of ROS with antioxidants promotes cone survival and function (Komeima et al. 2006; Komeima et al. 2007). The death of rods causes hyperoxia in the outer retina (Yu et al. 2000; Yu et al. 2004; Padnick-Silver et al. 2006) and hyperoxia causes photoreceptor cell death (Noell 1955; Yamada et al. 1999; Yamada et al. 2001; Wellard et al. 2005; Natoli et al. 2008). While it seems self-evident that increased tissue levels of oxygen would promote oxidative damage, it is not clear exactly how ROS are generated from excessive O2 in the retina. In this study, we explored whether two enzyme systems known to generate superoxide radicals are involved. We found that blockade of Nox reduced superoxide radicals in the outer retina of rd1+/+ mice as rods degenerated, reduced oxidative damage indicated by less carbonyl adducts on proteins, and promoted cone survival and function. Both m- and s-cone survival was improved as demonstrated by increased mRNA for m- and s-cone opsins in apocynin-treated mice. The rapidity of the rod degeneration in rd1+/+ mice leads to early onset and rapid progression of cone damage, consisting of loss of cone outer and inner segments followed later by cell death. Inner and outer segments are needed for cones to generate electrical activity from exposure to light, the basis of the ERG. The rapid loss of cone outer and inner segments in rd1+/+ mice makes it extremely difficult to have any impact at all on ERGs in this model, and therefore our most impressive finding is the significant preservation at P25 of photopic b-wave amplitude achieved by blockade of Nox. This was also found to occur in Q344ter mice, a model of dominant RP which also has a very rapid rate of degeneration. These findings indicate that one or more Nox enzymes play a critical role in the conversion of excess O2 in the retina into ROS that contributes to cone cell death in RP and hence blockade of Nox is an important new treatment strategy for RP.
The implication of Nox enzymes in cone cell death in RP is consistent with its role in other neurodegenerative diseases. The ROS generated by Nox and Duox enzymes function in cell signaling and other cellular activities, but just as hyperoxia in the outer retina can induce them to produce excessive amounts of ROS and contribute to tissue damage, other pathologic states can do likewise. Microglia have high levels of Nox2 which is increased in areas of brain inflammation and has been implicated in Alzheimer’s Disease, Parkinson’s Disease, and amyotrophic lateral sclerosis (Sankarapandi et al. 1998; Bianca et al. 1999; Green et al. 2001; Wyss-Coray and Mucke 2002; Wilkinson and Landreth 2006; Wu et al. 2006). Neurons and capillaries in brain express high levels of Nox4 which is upregulated by ischemia and may contribute to damage in stroke (Vallet et al. 2005).
Xanthine oxidase is another enzyme that generates ROS that has been implicated in oxidative damage in many tissues. (Chambers et al. 1985; Brown et al. 1988; Terada et al. 1992; Cappola et al. 2001; Patetsios et al. 2001) In rd1+/+ mice in which xanthine oxidase was blocked, high levels of superoxide radicals were still present in the outer retina and there was no beneficial effect on maintenance of cone survival or function. This indicates that xanthine oxidase does not contribute to generation of superoxide radicals when there is hyperoxia in the retina and is not a good target for therapeutic intervention in RP. In retrospect, this seems reasonable because the major source of xanthine oxidase in most tissue is vascular endothelium and the outer retina is avascular. In addition, the Xanthine oxidase promoter has a hypoxia response element and as a result it is upregulated by hypoxia and down-regulated by hyperoxia, (Terada et al. 1988) and thus even if it is present in the outer retina it would be down-regulated in the setting of RP.
The high level of oxygen in the outer retina that occurs as rods degenerate may activate Nox enzymes in the endogenous cells of the outer retina, cones and remaining rods, but may also activate the high levels of Nox2 in microglia, which have been demonstrated to migrate into the outer retina in RP (Thanos 1992; Gupta et al. 2003). The resulting increase in superoxide radicals can cause oxidative damage directly or indirectly through generation of even more damaging radicals. Nitric oxide (NO), which is present in high levels in the outer retina, rapidly reacts with superoxide radicals to form highly reactive and damaging peroxynitrite radicals. This is an important amplification step, because reduction of NO levels by inhibitors of NO synthase helps to preserve cone structure and function in models of RP.(Komeima et al. 2008) Ultimately a combination of agents including inhibitors of Nox and NOS along with antioxidants that directly scavenge ROS should be considered to test whether protection from oxidative damage can help maintain cone function in patients with RP.
In conclusion, our data suggest that Nox enzymes are key therapeutic targets in RP and that drugs that block Nox could be used to complement the use of antioxidants that directly scavenge ROS and NOS inhibitors that reduce a substrate for a particularly damaging ROS.
Acknowledgements
Supported by R01 EY05951 (PAC) from NIH and a gift from Dr. and Mrs. William Lake. Shinichi Usui is a Bausch and Lomb Japan Vitreoretinal Research Fellow and was supported by The Osaka Medical Research Foundation for Incurable Diseases. PAC is the George S. and Dolores Dore Eccles Professor of Ophthalmology and Neuroscience.
Abbreviations
- RP
Retinitis pigmentosa
- ERG
electroretinogram
- ROS
reactive oxygen species
- Nox
NADPH oxidase
- Phox
Nox of phagocytes
- Duox
dual oxidases
- PBS
phosphate-buffered saline
- PNA
peanut agglutinin
- NO
Nitric oxide
- P
postnatal day
References
- Al-Shabrawey M, Bartoli M, El-Remessy AB, Platt DH, Matragoon S, Behzadian MA, Caldwell RW, Caldwell RB. Inhibition of NAD(P)H oxidase activity blocks vascular endothelial growth factor overexpression and neovascularization during ischemic retinopathy. Am. J. Pathol. 2005;167:599–607. doi: 10.1016/S0002-9440(10)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin AJ, Boker T, Blumenroder SH, Lutz J, Spitznas M. Free radical scavenging and antioxidant activity of allopurinol and oxypurinol in experimental lens-induced uveitis. Invest. Ophthalmol. Vis. Sci. 1994;35:3897–3904. [PubMed] [Google Scholar]
- Babior BM, Lambeth JD, Nauseef W. The neutrophil NADPH oxidase. Arch. Biochem. Biophys. 2002;397:342–344. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2008;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- Bianca VD, Dusi S, Bianchini E, Dal Pra I, Rossi F. Beta-amyloid activates the O-2 froming NADPH oxidase in microglia, monocytes, and neutrophils. A possible inflammatory mechanism of neuronal damage in Alzheimer's disease. J. Biol. Chem. 1999;274:15493–15499. doi: 10.1074/jbc.274.22.15493. [DOI] [PubMed] [Google Scholar]
- Brown JM, Terada LS, Grosso MA, Whitmann GJ, Velasco SE, Patt A, Harken AH, Repine JE. Xanthine oxidase produces hydrogen peroxide which contributes to reperfusion injury of ischemic, isolated, perfused rat hearts. J. Clin. Invest. 1988;81:1297–1301. doi: 10.1172/JCI113448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder G, Heid HW, Jarasch ED, Mather IH. Immunological identification and determination of xanthine oxidase in cells and tissues. Differentiation. 1983;23:218–225. doi: 10.1111/j.1432-0436.1982.tb01286.x. [DOI] [PubMed] [Google Scholar]
- Cappola TP, Kass DA, Nelson GS, Berger RD, Rosas GO, Kobeissi ZA, Marban E, Hare JM. Allopurinol improves myocardial efficiency in patients with idiopathic dilated cardiomyopathy. Circulation. 2001;104:2407–2411. doi: 10.1161/hc4501.098928. [DOI] [PubMed] [Google Scholar]
- Carter-Dawson LD, LaVail MM, Sidman RL. Differential effects of the rd mutation on rods and cones in the mouse retina. Invest. Ophthalmol. Vis. Sci. 1978;17:489–498. [PubMed] [Google Scholar]
- Chambers DE, Parks DA, Patterson G, Roy R, McCord JM, Yoshida S, Parmley LF, Downey JM. Xanthine oxidase as a source of free radical damage in myocardial ischemia. J. Mol. Cell. Cardiol. 1985;17:145–152. doi: 10.1016/s0022-2828(85)80017-1. [DOI] [PubMed] [Google Scholar]
- Daiger SP, Bowne SJ, Sullivan LS. Perspective on genes and mutations causing retinitis pigmentosa. Arch. Ophthalmol. 2007;125:151–158. doi: 10.1001/archopht.125.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryja TP, McGee tL, Reichel E, Hahn LB, Cowley GS, Yandell DW, Sandberg MA, Berson EL. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature. 1990;343:364–366. doi: 10.1038/343364a0. [DOI] [PubMed] [Google Scholar]
- Green SP, Cairns B, Rae J, Errett-Baroncini C, Hongo JA, Erickson RW, Curnutte JT. Induction of gp91-phox, a component of the phagocyte NADPH oxidase, in microglial cells during centroal nervous system inflammation. J. Cerebral Blood Flow Met. 2001;21:374–384. doi: 10.1097/00004647-200104000-00006. [DOI] [PubMed] [Google Scholar]
- Gupta N, Brown KE, Milam AH. Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Exp. Eye Res. 2003;76:463–471. doi: 10.1016/s0014-4835(02)00332-9. [DOI] [PubMed] [Google Scholar]
- Hille R, Massey V. Studies on the oxidative half-reaction of xanthine oxidase. J. Biol. Chem. 1981;256:9090–9095. [PubMed] [Google Scholar]
- Hille R, Nishino T. Xanthine oxidase and xanthine dehydrogenase. FASEB J. 1995;9:995–1003. [PubMed] [Google Scholar]
- Isogai Y, Iizuka T, Shiro Y. The mechanism of electron donation to molecular oxygen by phagocytic cytochrome b558. J. Biol. Chem. 1995;270:7857–7857. doi: 10.1074/jbc.270.14.7853. [DOI] [PubMed] [Google Scholar]
- Jarasch ED, Grund C, Bruder G, Heid HW, Keenan TW, Franke WW. Localization of xanthine oxidase in mammary-gland epthelium and capillary endothelium. Cell. 1981;25:67–82. doi: 10.1016/0092-8674(81)90232-4. [DOI] [PubMed] [Google Scholar]
- Koenig R. Bardet-Biedl syndrome and Usher syndrome. Dev. Ophthalmol. 2003;37:126–140. doi: 10.1159/000072043. [DOI] [PubMed] [Google Scholar]
- Komeima K, Rogers BS, Campochiaro PA. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J. Cell. Physiol. 2007;213:809–815. doi: 10.1002/jcp.21152. [DOI] [PubMed] [Google Scholar]
- Komeima K, Rogers BS, Lu L, Campochiaro PA. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc. Natil. Acad. Sci. USA. 2006;103:11300–11305. doi: 10.1073/pnas.0604056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeima K, Usui S, Shen J, Rogers BS, Campochiaro PA. Blockade of neuronal nitric oxide synthase reduces cone cell death in a model of retinitis pigmentosa. Free Radic. Biol. Med. 2008;45:905–912. doi: 10.1016/j.freeradbiomed.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic. Biol. Med. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Dinculescu A, Shan Z, Miller R, Pang J, Lewin AS, Raizada MK, Hauswirth WW. Downregulation of p22phox in retinal pigment epithelial cells inhibits choroidal neovascularization in mice. Molec. Ther. 2008;16:1688–1694. doi: 10.1038/mt.2008.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder N, Rapola J, Raivio KO. Cellular expression of xanthine oxidoredutase protein in normal human tissues. Lab. Invest. 1999;79:967–974. [PubMed] [Google Scholar]
- Lu L, Oveson BC, Jo YJ, Lauer T, Usui S, Komeima K, Xie B, Campochiaro PA. Increased expression of glutathione peroxidase 4 strongly protects retina from oxidative damage. Antioxid. Redox. Signal. 2009;11:715–724. doi: 10.1089/ars.2008.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin ME, Sandberg MA, Berson EL, Dryja TP. Recessive mutations in the gene encoding the beta subunit of rod phosphodiesterase in patients with retinitis pigmenta. Nat. Genet. 1993;4:130–134. doi: 10.1038/ng0693-130. [DOI] [PubMed] [Google Scholar]
- Natoli R, Provis J, Valter K, Stone J. Expression and role of the early-response gene oxr1 in the hyperoxia-challenged mouse retina. Invest. Ophthalmol. Vis. Sci. 2008;49:4561–4567. doi: 10.1167/iovs.08-1722. [DOI] [PubMed] [Google Scholar]
- Noell WK. Visual cell effects of high oxygen pressures. Fed. Proc. 1955;14:107. [Google Scholar]
- Okoye G, Zimmer J, Sung J, P G, Deering T, Nambu N, Hackett SF, Melia M, Esumi N, Zack DJ, Campochiaro PA. Increased expression of BDNF preserves retinal function and slows cell death from rhodopsin mutation or oxidative damage. J. Neuosci. 2003;23:4164–4172. doi: 10.1523/JNEUROSCI.23-10-04164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padnick-Silver L, Derwent JJK, Giuliano E, Narfstrom K, Linsenmeier RA. Retinal oxygenation and oxygen metabolism in abyssinian cats with a hereditary retinal degeneration. Invest. Ophthalmol. Vis. Sci. 2006;47:3683–3689. doi: 10.1167/iovs.05-1284. [DOI] [PubMed] [Google Scholar]
- Patetsios P, Song M, Shutze WP, Pappas C, Rodino W, Ramirez JA, Panetta TF. Identification of uric acid and xanthine oxidase in atherosclerotic plaque. Am. J. Cardiol. 2001;88:188–191. doi: 10.1016/s0002-9149(01)01621-6. [DOI] [PubMed] [Google Scholar]
- Saito Y, Geisen P, Uppal A, Hartnett ME. Inhibition of NAD(P)H oxidase reduces apoptosis and avascular retina in an animal model of retinopathy of prematurity. Molec. Vision. 2007;13:840–853. [PMC free article] [PubMed] [Google Scholar]
- Sancho-Pelluz J, Arango-Gonzalez B, Kustermann S, Romero FJ, van Veen T, Zrenner E, Ekstrom P, Paquet-Durand F. Photoreceptor cell death mechanisms in inherited retinal degeneration. Mol. Neurobiol. 2008;38:253–269. doi: 10.1007/s12035-008-8045-9. [DOI] [PubMed] [Google Scholar]
- Sankarapandi S, Zweier JL, Mukherjee G, Quinn MT, Hsuo DL. Measurement and characterization of superoxide generation in microglial cells: evidence for an NADPH oxidase-dependent pathway. Arch. Biochem. Biophys. 1998;353:312–321. doi: 10.1006/abbi.1998.0658. [DOI] [PubMed] [Google Scholar]
- Shen J, Yan X, Dong A, Petters RM, Peng Y-W, Wong F, Campochiaro PA. Oxidative damage is a potential cause of cone cell death in retinitis pigmentosa. J. Cell. Physiol. 2005;203:457–464. doi: 10.1002/jcp.20346. [DOI] [PubMed] [Google Scholar]
- Stone J, Maslim J, Valter-Kocsi K, Mervin K, Bowers F, Chu Y, Barnett N, Provis J, Lewis G, Fisher SK, Bisti S, Gargini C, Cervetto L, Merin S, Pe'er J. Mechanisms of photoreceptor death and survival in mammalian retina. Prog. Ret. Eye Res. 1999;18:689–735. doi: 10.1016/s1350-9462(98)00032-9. [DOI] [PubMed] [Google Scholar]
- Sung C-H, Makino C, Baylor D, Nathans J. A rhodopsin gene mutation responsible for autosomal dominant retinitis pigmentosa results in a protein that is defective in localization to the photoreceptor outer segment. J. Neurosci. 1994;14:5818–5833. doi: 10.1523/JNEUROSCI.14-10-05818.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung C-H, Davenport CM, Hennessey JC, Maumenee IH, J JS, Heckenlively JR, Nowakowski R, Fishman G, Gouras P, Nathans J. Rhodopsin mutations in autosomal dominant retinitis pigmentosa. Proc. Natl. Acad. Sci. USA. 1991;88:6481–6485. doi: 10.1073/pnas.88.15.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada LS, Beehler CJ, Brown JM, Grosso MA, Harken AH, McCord JM, Repine JE. Hyperoxia and self- or neutrophil-generated O2 metabolites inactivate xanthine oxidase. J. Appl. Physiol. 1988;65:2349–2353. doi: 10.1152/jappl.1988.65.5.2349. [DOI] [PubMed] [Google Scholar]
- Terada LS, Guidot DM, Leff JA, Willingham IR, Hanley ME, Piermattei D, Repine JE. Hypoxia injures endothelial cells by increasing endogenous xanthine oxidase activity. Proc. Natl. Acad. Sci. USA. 1992;89:3362–3366. doi: 10.1073/pnas.89.8.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos S. Sick photoreceptors attract activated microglia from the ganglion cell layer: a model to study the inflammatory cascades in rats with inherited retinal dystrophy. Brain Res. 1992;588:21–28. doi: 10.1016/0006-8993(92)91340-k. [DOI] [PubMed] [Google Scholar]
- Turrens JF. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrens JF, Freeman BA, Levitt JG, Crapo JD. The effet of hyperoxia on superoxide production by lung submitochondrial particles. Arch. Biochem. Biophys. 1982;217:401–410. doi: 10.1016/0003-9861(82)90518-5. [DOI] [PubMed] [Google Scholar]
- Ueno S, Pease ME, Wersinger DMB, Masuda T, Vinores SA, Licht T, Zack DJ, Quigley H, Keshet E, Campochiaro PA. Prolonged blockade of VEGF family members does not cause identifiable damage to retinal neurons or vessels. J. Cell. Physiol. 2008;217:13–22. doi: 10.1002/jcp.21445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet P, Charnay Y, Steger K, Ogier-Denis E, Herrmann F, Michel JP, Szanto I. Neuronal expression of the NADPH oxidase NOX4, and its regulation in mouse experimental brain ischemia. Neuroscience. 2005;132:233–238. doi: 10.1016/j.neuroscience.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Wellard J, Lee D, Valter K, Stone J. Photoreceptors in the rat retina are specifically vulnerable to both hypoxia and hyperoxia. Visual Neurosci. 2005;22:501–507. doi: 10.1017/S0952523805224112. [DOI] [PubMed] [Google Scholar]
- Wilkinson BL, Landreth GE. The microglial NADPH oxidase complex as a source of oxidative stress in Alzheimer's disease. J. Neuroinflammation. 2006;3:30. doi: 10.1186/1742-2094-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DC, Re DB, Nagai M, Ischiropoulos H, Przedborski S. NADPH oxidase enzyme modulated motor neuron degeneration in amyotrophic lateral sclerosis mice. Proc. Natl. Acad. Sci. USA. 2006;103:12132–12137. doi: 10.1073/pnas.0603670103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease-A double edged sword. Neuron. 2002;35:419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- Yamada H, Yamada E, Hackett SF, Ozaki H, Okamoto N, Campochiaro PA. Hyperoxia causes decreased expression of vascular endothelial growth factor and endothelial cell apoptosis in adult retina. J. Cell. Physiol. 1999;179:149–156. doi: 10.1002/(SICI)1097-4652(199905)179:2<149::AID-JCP5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Yamada H, Yamada E, Ando A, Esumi N, Bora N, Saikia J, Sung C-H, Zack DJ, Campochiaro PA. FGF2 decreases hyperoxia-induced cell death in mice. J Am Pathlol. 2001;159:1113–1120. doi: 10.1016/S0002-9440(10)61787-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu DY, Cringle SJ, Su EN, Yu PK. Intraretinal oxygen levels before and after photoreceptor loss in the RCS rat. Invest. Ophthalmol. Vis. Sci. 2000;41:3999–4006. [PubMed] [Google Scholar]
- Yu DY, Cringle SJ, Valter K, Walsh N, Lee D, Stone J. Photoreceptor death, trophic factor expression, retinal oxygen status, and photoreceptor function in the P23H rat. Invest. Ophthalmol. Vis. Sci. 2004;45:2013–2019. doi: 10.1167/iovs.03-0845. [DOI] [PubMed] [Google Scholar]
- Al-Shabrawey M, Bartoli M, El-Remessy AB, Platt DH, Matragoon S, Behzadian MA, Caldwell RW, Caldwell RB. Inhibition of NAD(P)H oxidase activity blocks vascular endothelial growth factor overexpression and neovascularization during ischemic retinopathy. Am. J. Pathol. 2005;167:599–607. doi: 10.1016/S0002-9440(10)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin AJ, Boker T, Blumenroder SH, Lutz J, Spitznas M. Free radical scavenging and antioxidant activity of allopurinol and oxypurinol in experimental lens-induced uveitis. Invest. Ophthalmol. Vis. Sci. 1994;35:3897–3904. [PubMed] [Google Scholar]
- Babior BM, Lambeth JD, Nauseef W. The neutrophil NADPH oxidase. Arch. Biochem. Biophys. 2002;397:342–344. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2008;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- Bianca VD, Dusi S, Bianchini E, Dal Pra I, Rossi F. Beta-amyloid activates the O-2 froming NADPH oxidase in microglia, monocytes, and neutrophils. A possible inflammatory mechanism of neuronal damage in Alzheimer's disease. J. Biol. Chem. 1999;274:15493–15499. doi: 10.1074/jbc.274.22.15493. [DOI] [PubMed] [Google Scholar]
- Brown JM, Terada LS, Grosso MA, Whitmann GJ, Velasco SE, Patt A, Harken AH, Repine JE. Xanthine oxidase produces hydrogen peroxide which contributes to reperfusion injury of ischemic, isolated, perfused rat hearts. J. Clin. Invest. 1988;81:1297–1301. doi: 10.1172/JCI113448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder G, Heid HW, Jarasch ED, Mather IH. Immunological identification and determination of xanthine oxidase in cells and tissues. Differentiation. 1983;23:218–225. doi: 10.1111/j.1432-0436.1982.tb01286.x. [DOI] [PubMed] [Google Scholar]
- Cappola TP, Kass DA, Nelson GS, Berger RD, Rosas GO, Kobeissi ZA, Marban E, Hare JM. Allopurinol improves myocardial efficiency in patients with idiopathic dilated cardiomyopathy. Circulation. 2001;104:2407–2411. doi: 10.1161/hc4501.098928. [DOI] [PubMed] [Google Scholar]
- Carter-Dawson LD, LaVail MM, Sidman RL. Differential effects of the rd mutation on rods and cones in the mouse retina. Invest. Ophthalmol. Vis. Sci. 1978;17:489–498. [PubMed] [Google Scholar]
- Chambers DE, Parks DA, Patterson G, Roy R, McCord JM, Yoshida S, Parmley LF, Downey JM. Xanthine oxidase as a source of free radical damage in myocardial ischemia. J. Mol. Cell. Cardiol. 1985;17:145–152. doi: 10.1016/s0022-2828(85)80017-1. [DOI] [PubMed] [Google Scholar]
- Daiger SP, Bowne SJ, Sullivan LS. Perspective on genes and mutations causing retinitis pigmentosa. Arch. Ophthalmol. 2007;125:151–158. doi: 10.1001/archopht.125.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryja TP, McGee tL, Reichel E, Hahn LB, Cowley GS, Yandell DW, Sandberg MA, Berson EL. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature. 1990;343:364–366. doi: 10.1038/343364a0. [DOI] [PubMed] [Google Scholar]
- Green SP, Cairns B, Rae J, Errett-Baroncini C, Hongo JA, Erickson RW, Curnutte JT. Induction of gp91-phox, a component of the phagocyte NADPH oxidase, in microglial cells during centroal nervous system inflammation. J. Cerebral Blood Flow Met. 2001;21:374–384. doi: 10.1097/00004647-200104000-00006. [DOI] [PubMed] [Google Scholar]
- Gupta N, Brown KE, Milam AH. Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Exp. Eye Res. 2003;76:463–471. doi: 10.1016/s0014-4835(02)00332-9. [DOI] [PubMed] [Google Scholar]
- Hille R, Massey V. Studies on the oxidative half-reaction of xanthine oxidase. J. Biol. Chem. 1981;256:9090–9095. [PubMed] [Google Scholar]
- Hille R, Nishino T. Xanthine oxidase and xanthine dehydrogenase. FASEB J. 1995;9:995–1003. [PubMed] [Google Scholar]
- Isogai Y, Iizuka T, Shiro Y. The mechanism of electron donation to molecular oxygen by phagocytic cytochrome b558. J. Biol. Chem. 1995;270:7857–7857. doi: 10.1074/jbc.270.14.7853. [DOI] [PubMed] [Google Scholar]
- Jarasch ED, Grund C, Bruder G, Heid HW, Keenan TW, Franke WW. Localization of xanthine oxidase in mammary-gland epthelium and capillary endothelium. Cell. 1981;25:67–82. doi: 10.1016/0092-8674(81)90232-4. [DOI] [PubMed] [Google Scholar]
- Koenig R. Bardet-Biedl syndrome and Usher syndrome. Dev. Ophthalmol. 2003;37:126–140. doi: 10.1159/000072043. [DOI] [PubMed] [Google Scholar]
- Komeima K, Rogers BS, Campochiaro PA. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J. Cell. Physiol. 2007;213:809–815. doi: 10.1002/jcp.21152. [DOI] [PubMed] [Google Scholar]
- Komeima K, Rogers BS, Lu L, Campochiaro PA. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc. Natil. Acad. Sci. USA. 2006;103:11300–11305. doi: 10.1073/pnas.0604056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeima K, Usui S, Shen J, Rogers BS, Campochiaro PA. Blockade of neuronal nitric oxide synthase reduces cone cell death in a model of retinitis pigmentosa. Free Radic. Biol. Med. 2008;45:905–912. doi: 10.1016/j.freeradbiomed.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic. Biol. Med. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Dinculescu A, Shan Z, Miller R, Pang J, Lewin AS, Raizada MK, Hauswirth WW. Downregulation of p22phox in retinal pigment epithelial cells inhibits choroidal neovascularization in mice. Molec. Ther. 2008;16:1688–1694. doi: 10.1038/mt.2008.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder N, Rapola J, Raivio KO. Cellular expression of xanthine oxidoredutase protein in normal human tissues. Lab. Invest. 1999;79:967–974. [PubMed] [Google Scholar]
- Lu L, Oveson BC, Jo YJ, Lauer T, Usui S, Komeima K, Xie B, Campochiaro PA. Increased expression of glutathione peroxidase 4 strongly protects retina from oxidative damage. Antioxid. Redox. Signal. 2009;11:715–724. doi: 10.1089/ars.2008.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin ME, Sandberg MA, Berson EL, Dryja TP. Recessive mutations in the gene encoding the beta subunit of rod phosphodiesterase in patients with retinitis pigmenta. Nat. Genet. 1993;4:130–134. doi: 10.1038/ng0693-130. [DOI] [PubMed] [Google Scholar]
- Natoli R, Provis J, Valter K, Stone J. Expression and role of the early-response gene oxr1 in the hyperoxia-challenged mouse retina. Invest. Ophthalmol. Vis. Sci. 2008;49:4561–4567. doi: 10.1167/iovs.08-1722. [DOI] [PubMed] [Google Scholar]
- Noell WK. Visual cell effects of high oxygen pressures. Fed. Proc. 1955;14:107. [Google Scholar]
- Okoye G, Zimmer J, Sung J, P G, Deering T, Nambu N, Hackett SF, Melia M, Esumi N, Zack DJ, Campochiaro PA. Increased expression of BDNF preserves retinal function and slows cell death from rhodopsin mutation or oxidative damage. J. Neuosci. 2003;23:4164–4172. doi: 10.1523/JNEUROSCI.23-10-04164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padnick-Silver L, Derwent JJK, Giuliano E, Narfstrom K, Linsenmeier RA. Retinal oxygenation and oxygen metabolism in abyssinian cats with a hereditary retinal degeneration. Invest. Ophthalmol. Vis. Sci. 2006;47:3683–3689. doi: 10.1167/iovs.05-1284. [DOI] [PubMed] [Google Scholar]
- Patetsios P, Song M, Shutze WP, Pappas C, Rodino W, Ramirez JA, Panetta TF. Identification of uric acid and xanthine oxidase in atherosclerotic plaque. Am. J. Cardiol. 2001;88:188–191. doi: 10.1016/s0002-9149(01)01621-6. [DOI] [PubMed] [Google Scholar]
- Saito Y, Geisen P, Uppal A, Hartnett ME. Inhibition of NAD(P)H oxidase reduces apoptosis and avascular retina in an animal model of retinopathy of prematurity. Molec. Vision. 2007;13:840–853. [PMC free article] [PubMed] [Google Scholar]
- Sancho-Pelluz J, Arango-Gonzalez B, Kustermann S, Romero FJ, van Veen T, Zrenner E, Ekstrom P, Paquet-Durand F. Photoreceptor cell death mechanisms in inherited retinal degeneration. Mol. Neurobiol. 2008;38:253–269. doi: 10.1007/s12035-008-8045-9. [DOI] [PubMed] [Google Scholar]
- Sankarapandi S, Zweier JL, Mukherjee G, Quinn MT, Hsuo DL. Measurement and characterization of superoxide generation in microglial cells: evidence for an NADPH oxidase-dependent pathway. Arch. Biochem. Biophys. 1998;353:312–321. doi: 10.1006/abbi.1998.0658. [DOI] [PubMed] [Google Scholar]
- Shen J, Yan X, Dong A, Petters RM, Peng Y-W, Wong F, Campochiaro PA. Oxidative damage is a potential cause of cone cell death in retinitis pigmentosa. J. Cell. Physiol. 2005;203:457–464. doi: 10.1002/jcp.20346. [DOI] [PubMed] [Google Scholar]
- Stone J, Maslim J, Valter-Kocsi K, Mervin K, Bowers F, Chu Y, Barnett N, Provis J, Lewis G, Fisher SK, Bisti S, Gargini C, Cervetto L, Merin S, Pe'er J. Mechanisms of photoreceptor death and survival in mammalian retina. Prog. Ret. Eye Res. 1999;18:689–735. doi: 10.1016/s1350-9462(98)00032-9. [DOI] [PubMed] [Google Scholar]
- Sung C-H, Makino C, Baylor D, Nathans J. A rhodopsin gene mutation responsible for autosomal dominant retinitis pigmentosa results in a protein that is defective in localization to the photoreceptor outer segment. J. Neurosci. 1994;14:5818–5833. doi: 10.1523/JNEUROSCI.14-10-05818.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung C-H, Davenport CM, Hennessey JC, Maumenee IH, J JS, Heckenlively JR, Nowakowski R, Fishman G, Gouras P, Nathans J. Rhodopsin mutations in autosomal dominant retinitis pigmentosa. Proc. Natl. Acad. Sci. USA. 1991;88:6481–6485. doi: 10.1073/pnas.88.15.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada LS, Beehler CJ, Brown JM, Grosso MA, Harken AH, McCord JM, Repine JE. Hyperoxia and self- or neutrophil-generated O2 metabolites inactivate xanthine oxidase. J. Appl. Physiol. 1988;65:2349–2353. doi: 10.1152/jappl.1988.65.5.2349. [DOI] [PubMed] [Google Scholar]
- Terada LS, Guidot DM, Leff JA, Willingham IR, Hanley ME, Piermattei D, Repine JE. Hypoxia injures endothelial cells by increasing endogenous xanthine oxidase activity. Proc. Natl. Acad. Sci. USA. 1992;89:3362–3366. doi: 10.1073/pnas.89.8.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos S. Sick photoreceptors attract activated microglia from the ganglion cell layer: a model to study the inflammatory cascades in rats with inherited retinal dystrophy. Brain Res. 1992;588:21–28. doi: 10.1016/0006-8993(92)91340-k. [DOI] [PubMed] [Google Scholar]
- Turrens JF. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrens JF, Freeman BA, Levitt JG, Crapo JD. The effet of hyperoxia on superoxide production by lung submitochondrial particles. Arch. Biochem. Biophys. 1982;217:401–410. doi: 10.1016/0003-9861(82)90518-5. [DOI] [PubMed] [Google Scholar]
- Ueno S, Pease ME, Wersinger DMB, Masuda T, Vinores SA, Licht T, Zack DJ, Quigley H, Keshet E, Campochiaro PA. Prolonged blockade of VEGF family members does not cause identifiable damage to retinal neurons or vessels. J. Cell. Physiol. 2008;217:13–22. doi: 10.1002/jcp.21445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet P, Charnay Y, Steger K, Ogier-Denis E, Herrmann F, Michel JP, Szanto I. Neuronal expression of the NADPH oxidase NOX4, and its regulation in mouse experimental brain ischemia. Neuroscience. 2005;132:233–238. doi: 10.1016/j.neuroscience.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Wellard J, Lee D, Valter K, Stone J. Photoreceptors in the rat retina are specifically vulnerable to both hypoxia and hyperoxia. Visual Neurosci. 2005;22:501–507. doi: 10.1017/S0952523805224112. [DOI] [PubMed] [Google Scholar]
- Wilkinson BL, Landreth GE. The microglial NADPH oxidase complex as a source of oxidative stress in Alzheimer's disease. J. Neuroinflammation. 2006;3:30. doi: 10.1186/1742-2094-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DC, Re DB, Nagai M, Ischiropoulos H, Przedborski S. NADPH oxidase enzyme modulated motor neuron degeneration in amyotrophic lateral sclerosis mice. Proc. Natl. Acad. Sci. USA. 2006;103:12132–12137. doi: 10.1073/pnas.0603670103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease-A double edged sword. Neuron. 2002;35:419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- Yamada H, Yamada E, Hackett SF, Ozaki H, Okamoto N, Campochiaro PA. Hyperoxia causes decreased expression of vascular endothelial growth factor and endothelial cell apoptosis in adult retina. J. Cell. Physiol. 1999;179:149–156. doi: 10.1002/(SICI)1097-4652(199905)179:2<149::AID-JCP5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Yamada H, Yamada E, Ando A, Esumi N, Bora N, Saikia J, Sung C-H, Zack DJ, Campochiaro PA. FGF2 decreases hyperoxia-induced cell death in mice. J Am Pathlol. 2001;159:1113–1120. doi: 10.1016/S0002-9440(10)61787-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu DY, Cringle SJ, Su EN, Yu PK. Intraretinal oxygen levels before and after photoreceptor loss in the RCS rat. Invest. Ophthalmol. Vis. Sci. 2000;41:3999–4006. [PubMed] [Google Scholar]
- Yu DY, Cringle SJ, Valter K, Walsh N, Lee D, Stone J. Photoreceptor death, trophic factor expression, retinal oxygen status, and photoreceptor function in the P23H rat. Invest. Ophthalmol. Vis. Sci. 2004;45:2013–2019. doi: 10.1167/iovs.03-0845. [DOI] [PubMed] [Google Scholar]