Abstract
Atypical antipsychotic treatment has been associated with serious metabolic adverse events, such as glucose dysregulation and development of type 2 diabetes. As part of our studies on possible underlying mechanisms, we investigated the acute effects of various typical and atypical antipsychotics on plasma glucose and insulin in FVB/N mice, a strain that showed a more pronounced hyperglycemic response to clozapine than C57BL/6 and CD-1 mice. Acute administration of high doses of clozapine, olanzapine, quetiapine, perphenazine, or chlorpromazine significantly increased plasma glucose by 100%–140% above basal levels without significant effects on insulin levels. In contrast, risperidone reduced plasma glucose (−30%) and markedly enhanced plasma insulin levels. Doses of ziprasidone that gave 50-fold higher free plasma concentrations than therapeutic plasma levels, as well as high doses of aripiprazole and haloperidol, did not significantly alter either glucose or insulin levels. Clozapine- and olanzapine-induced hyperglycemia occurred at free plasma concentrations that were within, or one order of magnitude above, the range of therapeutic plasma levels. Pretreatment with either the ganglionic blocker hexamethonium, or the α2 adrenergic receptor antagonist yohimbine, blocked the clozapine- and chlorpromazine-induced increase in glucose levels. Taken together, these results suggest that typical and atypical antipsychotics with known metabolic liability produce acute hyperglycemia in mice and that this effect is likely driven by activation of the sympathetic autonomic nervous system via a central mechanism.
Keywords: antipsychotic, hyperglycemia, insulin, mouse
Introduction
Increasing clinical evidence suggests that antipsychotic drugs can elicit a range of metabolic side effects, such as treatment-associated weight gain, hyperglycemia, lipid abnormalities, and development of type 2 diabetes.1,2 Given these serious health risks, the US Food and Drug Administration requested a class labeling for all currently marketed second-generation (atypical) antipsychotics (clozapine, olanzapine, risperidone, quetiapine, ziprasidone, and aripiprazole) for an increased risk in developing diabetes. Clinical data, however, indicate a considerable variability in drug-induced metabolic abnormalities among these antipsychotics. A recent consensus statement concluded that clozapine and olanzapine are associated with the greatest risk for weight gain, type 2 diabetes, and dyslipidemia, while risperidone and quetiapine have intermediate weight gain effects and discrepant results regarding the risk for diabetes. Ziprasidone and aripiprazole, the 2 newest antipsychotic drugs on the market, have not been associated with weight gain or the development of diabetes.3 It should be mentioned that the adverse effects of first-generation typical antipsychotics (eg, chlorpromazine) on glucose homeostatis were known before the introduction of atypical antipsychotics.4
While weight gain, a well-documented side effect of long-term treatment with some antipsychotics,5 is a significant risk factor for type 2 diabetes, there is clinical evidence for increased diabetic liability independent of weight gain. Numerous reports have documented the rapid onset or exacerbation of diabetes and occurrence of hyperglycemic crises shortly after initiation of therapy with several atypical antipsychotics.6–8 At the molecular level, mechanisms responsible for the metabolic side effect of antipsychotics are poorly understood. In addition, receptor mechanisms that are responsible for weight gain may be different from those that cause insulin resistance and diabetes. This can obviously confound mechanistic studies on antipsychotic-induced hyperglycemia because weight gain itself can lead to insulin resistance. Investigations on diabetogenic mechanisms per se, independent of weight gain, are therefore preferably conducted in acute experiments, such as the present and our earlier studies,9,10 because these exclude any contributing factors related to changes in body composition.
Acute hyperglycemic effects of antipsychotics have been demonstrated in preclinical animal models for chlorpromazine in mice11,12 and rats13 and for various atypical antipsychotics in mice.14 In addition, a recent hyperinsulinemic, euglycemic clamp study in rats showed the rapid development of peripheral insulin resistance after acute clozapine and olanzapine administration.10 The aim of the present studies was to characterize the acute effects of various typical and atypical antipsychotics on plasma glucose and insulin levels in mice. To probe the mechanism of antipsychotic-induced hyperglycemia, the effects of pretreatment with the ganglionic blocker, hexamethonium, and the α2 adrenergic receptor antagonist, yohimbine, were examined. Free plasma concentrations of olanzapine, clozapine, and ziprasidone were determined to relate plasma levels in the mouse to clinically relevant drug exposures.
Methods
Animals
Male CD-1 (Charles River Breeding Laboratories Inc, Wilmington, MA), C57BL/6 (Jackson Laboratories, Bar Harbor, ME), and FVB/N (Taconic Farms Inc, Germantown, NY) mice, weighing 25–30 g were used. Animals were allowed at least 5 days of acclimatization to the vivarium with unlimited access to food (Purina 5001, PMI Nutrition International, St Louis, MO) and water prior to the study. Mice were fasted for about 18 hours prior to the start of the experiments but had free access to water at all times. Studies were conducted between 9:00 and 14:00 hours. All procedures were conducted in compliance with the Animal Welfare Act Regulations (9 Code of Federal Regulations Parts 1, 2, and 3) and with the “Guide for the Care and Use of Laboratory Animals” (Institute of Laboratory Animal Resources 1996) and were approved by the Institutional Animal Care and Use Committee.
Study Procedures
Mice (N = 5–10 per group) were randomly assigned to treatment conditions. Antipsychotic drugs were initially tested according to the procedure described by Dwyer and Donohue.14 After collection of a basal blood sample (100 μl), mice were injected intraperitoneally (i.p.) with vehicle or antipsychotic drug, and a second 100-μl blood sample was obtained 3 hours later. Blood samples were collected by retroorbital sinus bleeding (100 μl) using heparinized capillary tubes and then transferred to microfuge tubes containing 5 μl of 0.15 M sodium ethylenediamine tetraacetic acid. Plasma was separated by centrifugation (6000g, for 5 minutes) within 30 minutes of collection and divided for glucose and insulin measurements. In all subsequent experiments, collection of a basal blood sample was omitted to minimize stress. For dose-response studies with clozapine, olanzapine, and ziprasidone, a single blood sample (100 μl) was collected 1 hour postdosing via retroorbital sinus bleeding. Animals were then immediately euthanized with CO2, and an additional blood sample (0.5 ml) was collected via the inferior vena cava for drug level analysis. In experiments involving pretreatment with hexamethonium or yohimbine (5 minutes prior to antipsychotic drug), a single retroorbital blood sample (100 μl) was collected 1 hour following chlorpromazine, clozapine, or vehicle injections. For the glucose tolerance test, mice were given an intraperitoneal injection of glucose (1 mg/kg), and plasma glucose and insulin concentration were determined at 0, 15, and 45 minutes. At each time point, retroorbital blood samples (100 μl) were taken from a different cohort of mice.
Assays
For glucose measurements, 10 μl of plasma was added to 60 μl of 0.9% saline and immediately analyzed using the glucose hexokinase method (Roche 912 Clinical Autoanalyzer, Roche Diagnostics, Indianapolis, IN). For insulin measurements, 20–30 μl of plasma was added to microfuge tubes and frozen at −80°C until a mouse insulin enzyme-linked immunosorbent assay (ALPCO Diagnostics, Windham, NH) was performed. Inter- and intraassay coefficients of variation were 10.2% and 9.4%, respectively.
Plasma concentrations of clozapine, olanzapine, and ziprasidone were determined by high-performance liquid chromatography/mass spectrometry after liquid-liquid extraction, as described previously.15 Drug plasma protein binding in FVB/N mice was determined according to Maurer et al,16 and free plasma levels of clozapine, olanzapine, and ziprasidone were calculated using the unbound fractions (fuplasma).
Drugs
Haloperidol, clozapine, chlorpromazine, perphenazine, risperidone, hexamethonium, and yohimbine were purchased from Sigma Aldrich (St Louis, MO). d-Glucose was obtained from Fisher Scientific (Fair Lawn, NJ). Olanzapine, quetiapine, aripiprazole, and ziprasidone were synthesized at Pfizer Inc (Groton, CT). Antipsychotic drugs were dissolved in physiological saline containing 0.1% citric acid and 0.1%–0.5% of Cremophor EL (polyoxyethylene glycerol triricinoleate 35). Hexamethonium and yohimbine were dissolved in physiological saline. All drugs, vehicle, or saline were administered i.p. at a volume of 10 ml/kg. Control mice were injected with physiological saline containing 0.1% citric acid and 0.1%–0.5% of Cremophor EL and for the hexamethonium and yohimbine studies with physiological saline.
Statistics
Data were analyzed by 1-way or 2-way analyses of variance (ANOVAs), as appropriate, followed by Dunnett or Bonferroni multiple comparison tests.
Results
Effect of Clozapine on Plasma Glucose in 3 Mouse Strains
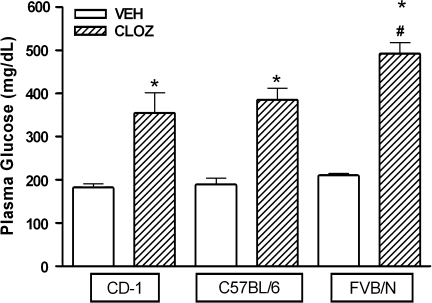
Fasted basal plasma glucose concentrations (mg/dl ± SEM, N = 6 per group) in the 3 mouse strains were not statistically different from each other: 198.3 ± 8.1 in CD-1 mice, 181.4 ± 10.2 in C57BL/6 mice, and 208.8 ± 6.7 in FVB/N mice (1-way ANOVA). Acute administration of clozapine (20 mg/kg, i.p.) induced marked increases in plasma glucose levels by 104%, 95%, and 134% compared with vehicle controls in CD-1, C57BL/6, and FVB/N mice, respectively (figure 1). Statistical analysis of the data with 2-way ANOVA revealed a significant effect of both the treatment (P < .0001) and strain (P < .01) on plasma glucose levels. Clozapine-induced increases in plasma glucose were significantly greater in FVB/N mice compared with C57BL/6 and CD-1 mice (P < .01, post hoc Bonferroni test). Given the greater response of FVB/N mice to clozapine, this strain was chosen for all subsequent studies.
Fig. 1.
Comparison of Clozapine Effects on Plasma Glucose in CD-1, C57BL/6, and FVB/N Mice. Plasma glucose concentrations (mg/dl) are shown at 3 h following vehicle (VEH) or clozapine (CLOZ, 20 mg/kg) administration. Data are presented as mean ± SEM (N = 6 per group). *P < .01 vs VEH, #P < .05 vs CD-1 CLOZ and C57BL/6 CLOZ (2-way analysis of variance, post hoc Bonferroni test).
Glucose Tolerance Test in FVB/N Mice
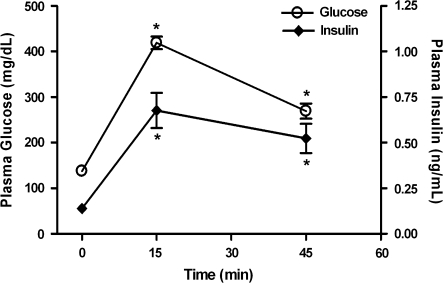
To demonstrate that glucose-insulin homeostasis is not impaired in FVB/N mice, glucose-stimulated insulin release was investigated using a glucose tolerance test (figure 2). Administration of 1 mg/kg glucose (i.p.) resulted in significant increases in both plasma glucose and insulin concentrations at 15 and 45 minutes postinjection compared with baseline (P < .01, 1-way ANOVA, post hoc Dunnett multiple comparison test).
Fig. 2.
Glucose Tolerance Test in FVB/N Mice. Time course of plasma glucose (mg/dl) and insulin (ng/ml) concentrations immediately prior to (t = 0 min) and following (t = 15 and 45 min) administration of glucose (1 mg/kg intraperitoneally). Data are presented as mean ± SEM (N = 5/group). *P < .01 vs 0 min glucose or insulin concentration. (1-way analysis of variance, post hoc Dunnett multiple comparison test).
Effect of Antipsychotics on Plasma Glucose and Insulin in FVB/N Mice
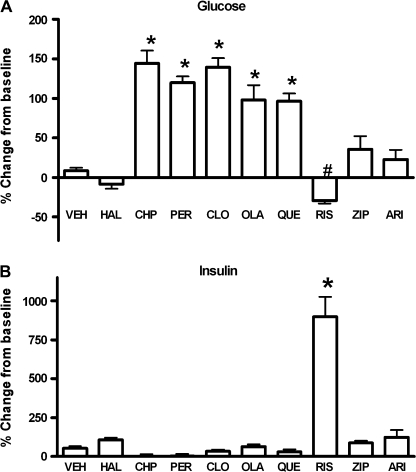
Effects of single, high doses of antipsychotic drugs on plasma glucose and insulin concentrations at 3 hours after dosing are expressed as percent change from baseline (N = 6 per group). The summary of the data obtained from several experiments is shown in figures 3A and 3B. There was no statistically significant difference between the vehicle-injected groups in these studies (1-way ANOVA); therefore, the average of the vehicle-treated controls is shown. Administration of the typical antipsychotics, chlorpromazine (10 mg/kg) and perphenazine (10 mg/kg), as well as the atypicals, clozapine (20 mg/kg), olanzapine (5 mg/kg), and quetiapine (10 mg/kg), induced robust increases in plasma glucose levels compared with their respective vehicle-injected controls (P < .01, 1-way ANOVA, post hoc Dunnett multiple comparison test). In contrast, haloperidol (2 mg/kg), aripiprazole (20 mg/kg), and ziprasidone (10 mg/kg) had no significant effect on glucose levels. Administration of risperidone (2 mg/kg) resulted in a small (30%), but statistically significant, decrease in plasma glucose levels (P < .05, 1-way ANOVA, post hoc Dunnett multiple comparison test). Risperidone treatment also caused a pronounced, more than 8-fold increase increase in plasma insulin (P < .01, 1-way ANOVA, post hoc Dunnett multiple comparison test). No statistically significant effects on insulin were observed in the other treatment groups compared with vehicle controls.
Fig. 3.
Effect of Various Antipsychotics on Plasma Glucose (A) and Insulin (B) in FVB/N Mice. Drugs were administered intraperitoneally as shown: vehicle (VEH), haloperidol (HAL, 2 mg/kg), chlorpromazine (CHP, 10 mg/kg), perphenazine (PER, 10 mg/kg), clozapine (CLO, 20 mg/kg), olanzapine (OLA, 5 mg/kg), quetiapine (QUE, 10 mg/kg), risperidone (RIS, 2 mg/kg), ziprasidone (ZIP, 10 mg/kg), and aripiprazole (ARI, 20 mg/kg). Data are shown as percent change at 3 h postdrug from baseline (mean ± SEM, N = 6–10/group). *P < .01 vs VEH (1-way analysis of variance, post hoc Dunnett multiple comparison test).
Dose-Response Effects of Clozapine, Olanzapine, and Ziprasidone on Plasma Glucose in FVB/N Mice: Correlation With Drug Exposure
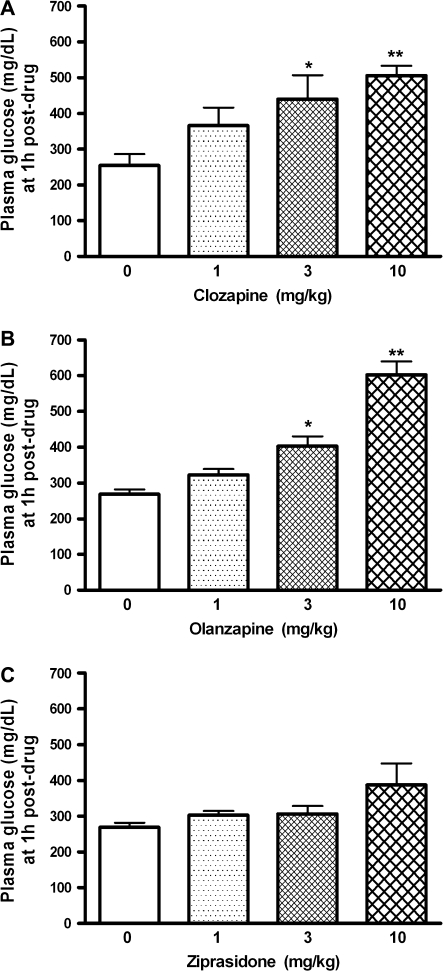
To study the dose dependency of the response and the drug levels associated with active doses, mice were treated with increasing doses of clozapine, olanzapine, or ziprasidone, and both plasma glucose and plasma drug concentrations were measured 1 hour after drug administration. Clozapine (1, 3, and 10 mg/kg, i.p.) treatment resulted in a dose-dependent increase in glucose levels by 43%, 72%, and 98% above vehicle controls, respectively (figure 4A). Statistical analysis revealed significant effects on glucose levels of the 3 (P < .05) and 10 mg/kg (P < .01) doses of clozapine vs vehicle treatment (1-way ANOVA, post hoc Dunnett multiple comparison test). Olanzapine (1, 3, and 10 mg/kg, i.p.) treatment resulted in comparable dose-dependent increases in glucose levels by 20%, 50%, and 124% above vehicle controls, respectively (figure 4B), with both 3 mg/kg– and 10 mg/kg–treated groups significantly different from vehicle treatment (P < .01, 1-way ANOVA, post hoc Dunnett multiple comparison test). In contrast to clozapine and olanzapine, ziprasidone (1, 3, and 10 mg/kg) did not significantly alter plasma glucose levels in mice producing a 12%, 13%, and 44% increase in glucose, respectively (figure 4C) above vehicle-injected controls.
Fig. 4.
Effect of Clozapine (A), Olanzapine (B), and Ziprasidone (C) on Plasma Glucose (mg/dl) Concentrations in FVB/N Mice. Drugs or vehicle (0) were administered 1 h prior to blood sample collection. Data are mean ± SEM (N = 6 per group). *P < .05, **P < .01 vs vehicle (1-way analysis of variance, post hoc Dunnett multiple comparison test).
Plasma concentrations of clozapine, olanzapine, and ziprasidone (1, 3, and 10 mg/kg) at 1 hour after dosing show that all 3 drugs exhibited dose-related nonlinear pharmacokinetics (table 1). Free plasma drug concentrations in mice were calculated using fuplasma 0.038, 0.147, and 0.050 for clozapine, olanzapine, and ziprasidone, respectively. Unbound drug plasma concentrations in mice at doses that produced hyperglycemia were in the same range as the unbound therapeutic steady state Cmax (maximum drug concentration in plasma) for clozapine, and 2- to 30-fold higher than therapeutic plasma levels for olanzapine (table 1). The highest dose of ziprasidone did not increase glucose levels yet produced free plasma drug levels in mice that were about 50-fold higher than free human therapeutic ziprasidone levels (table 1).
Table 1.
Plasma Concentrations of Clozapine, Olanzapine, and Ziprasidone in FVB/N Mice: Comparison With Therapeutically Relevant Plasma Drug Exposures
| Plasma Drug Concentrations (ng/ml ± SEM) |
||||||
| Clozapine |
Olanzapine |
Ziprasidone |
||||
| Total | Unbound | Total | Unbound | Total | Unbound | |
| Mouse | ||||||
| 1 mg/kg | 25.3 ± 4.9 | 1.0 ± 0.2 | 42.3 ± 6.9 | 6.2 ± 1.0 | 28.0 ± 3.1 | 1.4 ± 0.2 |
| 3 mg/kg | 133.9 ± 21.5 | 5.1 ± 0.8 | 220.5 ± 35.8 | 32.3 ± 5.3 | 85.7 ± 11.7 | 4.3 ± 0.6 |
| 10 mg/kg | 510.4 ± 37.2 | 19.4 ± 1.4 | 615.6 ± 71.9 | 91.1 ± 9.4 | 209.9 ± 58.8 | 10.5 ± 2.9 |
| Humana | ||||||
| Steady state Cmax | 350–420 | 19–23 | 45 | 3.2 | 202 | 0.2 |
Note: Unbound plasma concentrations in FVB/N mice were calculated using unbound fractions (fuplasma) 0.038, 0.147, and 0.050 for clozapine, olanzapine, and ziprasidone, respectively.
Human steady state Cmax (maximum drug concentration in plasma) values noted here are those reported at recommended clinical doses: clozapine 300–450 mg/day, olanzapine 12 mg/day, ziprasidone 80 mg bid. Human unbound plasma concentrations were calculated using fuplasma 0.055, 0.07, and 0.001 for clozapine, olanzapine, and ziprasidone, respectively, based on the following references: Schaber et al,38 Kassahun et al,39 and Wilner et al.40
Effect of Hexamethonium Pretreatment on Clozapine- and Chlorpromazine-Induced Hyperglycemia in FVB/N Mice
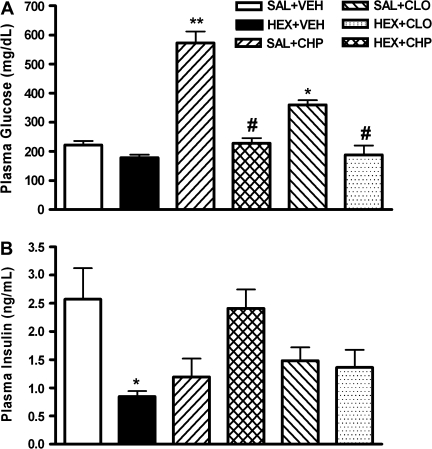
The ganglionic blocker hexamethonium was used to examine the involvement of the autonomic nervous system in antipsychotic-induced hyperglycemia. Animals were pretreated with 50 mg/kg hexamethonium 5 minutes prior to chlorpromazine or clozapine administration. When given alone, chlorpromazine (10 mg/kg) or clozapine (20 mg/kg) significantly increased plasma glucose levels, but pretreatment with hexamethonium completely prevented the hyperglycemic response induced by these drugs (figure 5A). Administration of chlorpromazine and clozapine, either alone or combined with hexamethonium, did not significantly change plasma insulin concentrations compared with vehicle controls (figure 5B). Hexamethonium plus vehicle treatment did not affect plasma glucose but caused a significant decrease in insulin levels (figures 5A and 5B).
Fig. 5.
Effect of Hexamethonium on Chlorpromazine- and Clozapine-Induced Hyperglycemia. Hexamethonium (HEX, 50 mg/kg) or saline (SAL) was administered 5 min prior to vehicle (VEH), chlorpromazine (CHP, 10 mg/kg), or clozapine (CLO, 20 mg/kg). Plasma glucose (A) and insulin (B) were determined 1 h after the last injection. Data are mean ± SEM (N = 5–6 per group). **P < .001 vs SAL + VEH, *P < .01 vs SAL + VEH, #P < .001 vs SAL + CHP or SAL + CLO (1-way analysis of variance, post hoc Bonferroni).
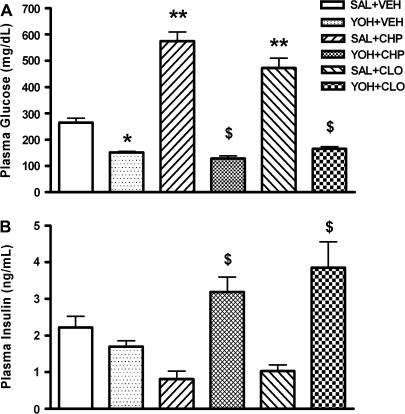
Effect of Yohimbine Pretreatment on Chlorpromazine- and Clozapine-Induced Hyperglycemia in FVB/N Mice
The α2 adrenoceptor antagonist yohimbine was used to examine the involvement of the sympathetic nervous system in the antipsychotic-induced hyperglycemia. Mice were pretreated with 4 mg/kg yohimbine 5 minutes prior to chlorpromazine or clozapine administration. Yohimbine pretreatment completely abolished the increases in plasma glucose produced by chlorpromazine (10 mg/kg) or clozapine (20 mg/kg) administration (figure 6A). Yohimbine alone caused a small, but statistically significant, reduction in plasma glucose (figure 6A). While the administration of yohimbine plus vehicle had no effect on insulin, the combination of yohimbine plus chlorpromazine or clozapine significantly increased insulin plasma levels (figure 6B).
Fig. 6.
Effect of Yohimbine on Chlorpromazine- and Clozapine-Induced Hyperglycemia. Yohimbine (YOH, 4 mg/kg) or saline (SAL) was administered 5 min prior to vehicle (VEH), chlorpromazine (CHP, 10 mg/kg), or clozapine (CLO, 20 mg/kg). Plasma glucose (A) and insulin (B) were determined 1 h after the last injection. Data are mean ± SEM (N = 5–6 per group). **P < .001 vs SAL + VEH, *P < .01 vs SAL + VEH, $P < .001 vs SAL + CHP or SAL + CLO (1-way analysis of variance, post hoc Bonferroni).
Discussion
This study investigated the effects of acute systemic administration of various antipsychotic drugs on plasma glucose and insulin levels in mice. In agreement with previous results in C57BL/6 mice,14 we found that the administration of a single, high dose of clozapine (20 mg/kg) induced a large, nearly 2-fold, elevation of plasma glucose levels in C57BL/6, CD-1, and FVB/N mice. While clozapine markedly increased glucose in all 3 mouse strains, the hyperglycemic response was significantly greater in the FVB/N strain compared with CD-1 and C57BL/6 mice. Although pharmacokinetic differences between these mouse strains cannot be excluded, the more pronounced hyperglycemic response to clozapine in FVB/N mice might be attributable to a dysregulation of autonomic nervous system activity.17 Interestingly, this strain has been shown to be a better genetic background than C57BL/6 for ob/ob mice in producing a more severe diabetic phenotype.18 These observations, together with our finding that the insulin response is not impaired in the glucose tolerance test, support the utility of FVB/N mice as a sensitive model for antipsychotic-induced diabetic liability.
Our results show that the typical antipsychotics, chlorpromazine and perphenazine, as well as the atypicals, olanzapine, clozapine, and quetiapine, elevated plasma glucose levels in FVB/N mice, confirming and extending previous findings.14 In addition, we found that high doses of other typical (haloperidol) and atypical antipsychotics (risperidone, aripiprazole, and ziprasidone) were devoid of a hyperglycemic effect. Risperidone appeared to be distinct from the other drugs because it significantly reduced glucose levels through induction of insulin release. This effect could be due to its potent α2 adrenoceptor antagonist activity19 because coadministration of the α2 adrenoceptor antagonist yohimbine with antipsychotic treatment also induced marked insulin release and thus prevented hyperglycemia (see below), although yohimbine alone had no effect on insulin. While the link between the acute glycemic effects in mice and drug-induced metabolic changes leading to diabetes and/or obesity in patients is complex and not well understood, our data show a good correlation between hyperglycemic effects in mice and diabetic liability in the clinic. Thus, those antipsychotics that have been shown to be diabetogenic in the clinic, such as clozapine, olanzapine,3 and chlorpromazine,4 induce a robust increase in glucose levels, while antipsychotics without diabetic liability such as ziprasidone, aripiprazole,3 and haloperidol20 do not affect plasma glucose. For compounds of intermediate diabetic risk, such as risperidone and quetiapine,3 glycemic effects in mice appear to be somewhat inconsistent. While quetiapine significantly increased plasma glucose, risperidone (2 mg/kg) decreased glucose levels in FVB/N mice, which is at odds with reports showing that risperidone induced hyperglycemia at 2 mg/kg, but not at 20 mg/kg, in C57BL/6 mice.14 This discrepancy could be due to the use of different mouse strains and/or experimental conditions.
The lack of change in insulin levels after chlorpromazine, perphenazine, clozapine, olanzapine, or quetiapine treatment suggests that these drugs effectively block the acute insulin secretory compensation mechanism. This is in excellent agreement with recent evidence from preclinical studies that inadequate compensatory insulin secretion plays a role in olanzapine-induced insulin resistance in dogs21 and that olanzapine and clozapine (3.2–10 mg/kg), but not ziprasidone (32 mg/kg) and risperidone (2 mg/kg), acutely induce profound insulin resistance in rats.10 The fact that acute risperidone treatment does not affect insulin sensitivity in dogs21 and rats10 is consistent with the pronounced increase in plasma insulin levels that we observed after risperidone administration.
Early studies investigating the glycemic effect of chlorpromazine in rats revealed that both systemic22 and intracerebroventricular23 drug administration resulted in acute hyperglycemia in intact, but not adrenalectomized, rats, indicating that chlorpromazine-induced hyperglycemia is centrally mediated and involves secretion of epinephrine from the adrenal medulla. The release of medullary epinephrine is controlled by sympathetic preganglionic (cholinergic) neurons and thus could be blocked by the ganglionic blocker hexamethonium. Our finding that pretreatment with hexamethonium completely inhibited the elevation of plasma glucose induced by either chlorpromazine or clozapine confirms and extends earlier data on the effects of combined chlorpromazine and hexamethonium administration in mice12 and rats.23 In the present studies, chlorpromazine- and clozapine-induced hyperglycemia in mice was also completely blocked by pretreatment with the α2 adrenoceptor antagonist, yohimbine, suggesting that the hyperglycemic effect is due to increased sympathetic nervous system activity. Such a mechanism has been hypothesized by Nakadate et al12 to underlie the hyperglycemic effect of chlorpromazine, and our data now provide evidence that this mechanism likely applies to atypical antipsychotics as well. Enhanced adrenergic activity is further supported by the fact that clozapine and risperidone, but not haloperidol, increase circulating norepinephrine levels in schizophrenic patients.24–26 The finding that in control animals hexamethonium reduces plasma insulin without affecting glucose, while yohimbine reduces plasma glucose without affecting insulin, suggests that glucose levels are mainly affected by epinephrine via sympathetic stimulation, whereas insulin regulation is more complex, involving, among others, cholinergic parasympathetic input. The fact that coadministering yohimbine with clozapine or chlorpromazine has the same effect as risperidone alone, ie, increased plasma insulin and normalized plasma glucose, indicates that α2 adrenoceptor blockade can relieve tonic suppression of insulin secretion. These data suggest that antipsychotic-induced hyperglycemia could be, at least in part, attributed to increased adrenergic activity via the sympathetic nervous system, but additional receptor mechanisms are likely to be involved.
The role of the autonomic nervous system in regulating blood glucose levels within a narrow range via complex central and peripheral mechanisms is well established. The autonomic nervous system innervates and regulates the functions of organs and tissues involved in energy metabolism, including liver, pancreas, skeletal muscle, and adipose tissues.27 Therefore, alteration or disruption of the regulation of this system could affect glucose balance via multiple mechanisms, such as changes in pancreatic insulin release, hepatic glucose output, and/or peripheral glucose utilization. Furthermore, the use of antipsychotic medications is associated with perturbation of the secretion and action of several hormones known to regulate glucose and fat metabolism (eg, glucagon, leptin, adiponectin, ghrelin).28 Further studies are required to determine the effect of acute antipsychotic drug administration on insulin counterregulatory hormones in mice and any possible role of sympathetic activation in such effects.
The receptor mechanisms responsible for the metabolic effects of antipsychotics are not well understood. Typical and atypical antipsychotics bind with high affinity to a wide variety of neurotransmitter receptors and transporters that could be involved in metabolic side effects. Evidence from pharmacological, genetic, and correlation analysis studies has identified a number of candidate mechanisms, eg, histamine H1 receptor,29,30 5-HT2C receptor,31,32 muscarinic3 acetylcholine receptor,9 and glucose transporters (GLUT),33 that could play a role in glucose dysregulation and/or weight gain. In addition, reduced cerebral glucose utilization was observed with chlorpromazine that has been ascribed to its direct actions on membrane permeability34,35 leading to possible impairment of glucose sensing in the brain. Although our data do not allow conclusions with regard to the specific receptor mechanisms involved in the acute glucose response, they help to rule out some potential underlying mechanisms. Thus, it is unlikely that effects on peripheral glucose transporters (GLUT1, GLUT4) found in pancreas, liver, muscle, or fat tissue play a significant role because our results suggest that the primary action of antipsychotics is likely to be central to the autonomic ganglion. This is supported by the fact that the most potent inhibitor of GLUT, ziprasidone,36 did not produce hyperglycemia in the FVB/N mouse and that olanzapine did not inhibit glucose transport in vitro, in vivo, or ex vivo.11,37 In addition, therapeutic plasma levels of clozapine and olanzapine are several orders of magnitude below their glucose transport IC50 values (concentrations required for 50% inhibition).36
Only a few preclinical studies on the adverse metabolic effects of antipsychotics have addressed how the doses and concentrations employed in animal or in vitro studies relate to therapeutic drug exposures in humans. In the clinic, antipsychotics are used under multiple-dosing conditions, often in combination with other drugs, and the resulting steady state concentrations cannot easily be reproduced in animal studies. However, the assessment of unbound plasma drug concentrations is a viable approach to relate hyperglycemic drug effects in mice to clinically relevant exposures (table 1). Results of our dose-response studies showing that ziprasidone did not induce hyperglycemia at any of the doses tested, while olanzapine and clozapine caused marked elevation in plasma glucose, further highlighted the distinct hyperglycemic liabilities of these compounds. The highest dose of ziprasidone (10 mg/kg) achieved plasma-free drug concentrations in the mouse (11.5 ng/ml) that exceeded 50-fold the clinically relevant drug exposures in man (0.2 ng/ml), indicating that ziprasidone is devoid of significant hyperglycemic liability. In contrast, hyperglycemic doses of clozapine yielded plasma-free drug concentrations (5–20 ng/ml) that were within the range of free therapeutic levels (19–23 ng/ml), while hyperglycemic effects of olanzapine occurred at free drug concentration in mice that were about 10-fold higher than free therapeutic human plasma levels reported for 12 mg/day olanzapine. It should be noted that in clinical practice olanzapine is often dosed up to 30 mg/day2 and that human plasma levels can thus be closer to plasma levels associated with hyperglycemia in mice.
In conclusion, we have shown that there is a good correlation between hyperglycemic effects in FVB/N mice and clinical diabetic liability of antipsychotics and that the FVB/N mouse strain could serve as a sensitive preclinical model to study the acute metabolic effects of antipsychotics. Our data also indicate that, regardless of their classification as typical or atypical, antipsychotic drugs display differential effects on plasma glucose that is likely to be related to their unique pharmacological properties. Finally, blockade of antipsychotic-induced hyperglycemia by a ganglion blocker or an α2 adrenoceptor antagonist in mice suggests that these drugs may elevate blood glucose via a central mechanism involving the sympathetic autonomic nervous system.
References
- 1.Newcomer JW. Abnormalities of glucose metabolism associated with atypical antipsychotic drugs. J Clin Psychiatry. 2004;65:36–46. [PubMed] [Google Scholar]
- 2.Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27:596–601. doi: 10.2337/diacare.27.2.596. Also published in J Clin Psychiatry. 2004;65:267–272. [DOI] [PubMed] [Google Scholar]
- 4.Erle G, Basso M, Federspil G, Sicolo N, Scandellari C. Effect of chlorpromazine on blood glucose and plasma insulin in man. Eur J Clin Pharmacol. 1977;11:15–18. doi: 10.1007/BF00561782. [DOI] [PubMed] [Google Scholar]
- 5.Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19:1–93. doi: 10.2165/00023210-200519001-00001. [DOI] [PubMed] [Google Scholar]
- 6.Hagg S, Joelsson L, Mjorndal T, Spigset O, Oja G, Dahlqvist R. Prevalence of diabetes and impaired glucose tolerance in patients treated with clozapine compared with patients treated with conventional depot neuroleptic medications. J Clin Psychiatry. 1998;59:294–299. doi: 10.4088/jcp.v59n0604. [DOI] [PubMed] [Google Scholar]
- 7.Koller E, Schneider B, Bennett K, Dubitsky G. Clozapine-associated diabetes. Am J Med. 2001;111:716–723. doi: 10.1016/s0002-9343(01)01000-2. [DOI] [PubMed] [Google Scholar]
- 8.Wilson DR, D'Souza L, Sarkar N, Newton M, Hammond C. New-onset diabetes and ketoacidosis with atypical antipsychotics. Schizophr Res. 2003;59:1–6. doi: 10.1016/s0920-9964(01)00331-0. [DOI] [PubMed] [Google Scholar]
- 9.Johnson DE, Yamazaki H, Ward KM, et al. Inhibitory effects of antipsychotics on carbachol-enhanced insulin secretion from perifused rat islets: role of muscarinic antagonism in antipsychotic-induced diabetes and hyperglycemia. Diabetes. 2005;54:1552–1558. doi: 10.2337/diabetes.54.5.1552. [DOI] [PubMed] [Google Scholar]
- 10.Houseknecht KL, Robertson AS, Zavadoski W, Gibbs EM, Johnson DE, Rollema H. Acute effects of atypical antipsychotics on whole-body insulin resistance in rats: implications for adverse metabolic effects. Neuropsychopharmacology. 2007;32:289–297. doi: 10.1038/sj.npp.1301209. [DOI] [PubMed] [Google Scholar]
- 11.Nakadate T, Kubota K, Muraki T, Kato R. Mechanism of chlorpromazine action on plasma glucose and cyclic AMP levels. Eur J Pharmacol. 1980;64:107–113. doi: 10.1016/0014-2999(80)90033-3. [DOI] [PubMed] [Google Scholar]
- 12.Nakadate T, Muraki T, Kato R. Effects of alpha- and beta-adrenergic blockers on chlorpromazine-induced elevation of plasma glucose and cyclic AMP in fed mice. Jpn J Pharmacol. 1980;30:199–206. doi: 10.1254/jjp.30.199. [DOI] [PubMed] [Google Scholar]
- 13.Bugajski J, Lech J. Effects of neuroleptics on blood glucose, free fatty acids and liver glycogen levels in the rat. Pol J Pharmacol Pharm. 1979;31:45–58. [PubMed] [Google Scholar]
- 14.Dwyer DS, Donohoe D. Induction of hyperglycemia in mice with atypical antipsychotic drugs that inhibit glucose uptake. Pharmacol Biochem Behav. 2003;75:255–260. doi: 10.1016/s0091-3057(03)00079-0. [DOI] [PubMed] [Google Scholar]
- 15.Johnson DE, Nedza FM, Spracklin DK, et al. The role of muscarinic receptor antagonism in antipsychotic-induced hippocampal acetylcholine release. Eur J Pharmacol. 2005;506:209–219. doi: 10.1016/j.ejphar.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Maurer TS, Debartolo DB, Tess DA, Scott DO. Relationship between exposure and nonspecific binding of thirty-three central nervous system drugs in mice. Drug Metab Dispos. 2005;33:175–181. doi: 10.1124/dmd.104.001222. [DOI] [PubMed] [Google Scholar]
- 17.Shusterman V, Usiene I, Harrigal C, et al. Strain-specific patterns of autonomic nervous system activity and heart failure susceptibility in mice. Am J Physiol Heart Circ Physiol. 2002;282:2076–2083. doi: 10.1152/ajpheart.00917.2001. [DOI] [PubMed] [Google Scholar]
- 18.Haluzik M, Colombo C, Gavrilova O, et al. Genetic background (C57BL/6J versus FVB/N) strongly influences the severity of diabetes and insulin resistance in ob/ob mice. Endocrinology. 2004;145:3258–3264. doi: 10.1210/en.2004-0219. [DOI] [PubMed] [Google Scholar]
- 19.Leysen JE, Gommeren W, Eens A, de Chaffoy de Courcelles D, Stoof JC, Janssen PA. Biochemical profile of risperidone, a new antipsychotic. J Pharmacol Exp Ther. 1988;247:661–670. [PubMed] [Google Scholar]
- 20.Newcomer JW, Haupt DW, Fucetola R, et al. Abnormalities in glucose regulation during antipsychotic treatment of schizophrenia. Arch Gen Psychiatry. 2002;59:337–345. doi: 10.1001/archpsyc.59.4.337. [DOI] [PubMed] [Google Scholar]
- 21.Ader M, Kim SP, Catalano KJ, et al. Metabolic dysregulation with atypical antipsychotics occurs in the absence of underlying disease: a placebo-controlled study of olanzapine and risperidone in dogs. Diabetes. 2005;54:862–871. doi: 10.2337/diabetes.54.3.862. [DOI] [PubMed] [Google Scholar]
- 22.Ghafghazi T, Miya TS, Mennear JH, Chalmers RK. Chlorpromazine and epinephrine hyperglycemic mechanisms. J Pharm Sci. 1968;57:1690–1693. doi: 10.1002/jps.2600571012. [DOI] [PubMed] [Google Scholar]
- 23.Fujimori K, Iwamoto T. Mechanisms of hyperglycemic response to chlorpromazine administered into lateral ventricle in rats. II. Secretion of epinephrine from adrenal medulla. Neuropharmacology. 1974;13:255–260. doi: 10.1016/0028-3908(74)90075-6. [DOI] [PubMed] [Google Scholar]
- 24.Breier A, Buchanan RW, Waltrip RW, Listwak S, Holmes C, Goldstein DS. The effect of clozapine on plasma norepinephrine: relationship to clinical efficacy. Neuropsychopharmacology. 1994;10:1–7. doi: 10.1038/npp.1994.1. [DOI] [PubMed] [Google Scholar]
- 25.Brown AS, Gewirtz G, Harkavy-Friedman J, et al. Effects of clozapine on plasma catecholamines and relation to treatment response in schizophrenia: a within-subject comparison with haloperidol. Neuropsychopharmacology. 1997;17:317–325. doi: 10.1016/S0893-133X(97)00073-0. [DOI] [PubMed] [Google Scholar]
- 26.See RE, Fido AA, Maurice M, Ibrahim MM, Salama GM. Risperidone-induced increase of plasma norepinephrine is not correlated with symptom improvement in chronic schizophrenia. Biol Psychiatry. 1999;45:1653–1656. doi: 10.1016/s0006-3223(98)00199-1. [DOI] [PubMed] [Google Scholar]
- 27.Nonogaki K. New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia. 2000;43:533–549. doi: 10.1007/s001250051341. [DOI] [PubMed] [Google Scholar]
- 28.Elias AN, Hofflich H. Abnormalities in glucose metabolism in patients with schizophrenia treated with atypical antipsychotic medications. Am J Med. 2008;121:98–104. doi: 10.1016/j.amjmed.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 29.Sakata T, Ookuma K, Fujimoto K, Fukagawa K, Yoshimatsu H. Histaminergic control of energy balance in rats. Brain Res Bull. 1991;27:371–375. doi: 10.1016/0361-9230(91)90127-6. [DOI] [PubMed] [Google Scholar]
- 30.Wirshing DA, Wirshing WC, Kysar L, et al. Novel antipsychotics: comparison of weight gain liabilities. J Clin Psychiatry. 1999;60:358–363. [PubMed] [Google Scholar]
- 31.Tecott LH, Sun LM, Akana SF, et al. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995;374:542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- 32.Gilles M, Wilke A, Kopf D, Nonell A, Lehnert H, Deuschle M. Antagonism of the serotonin (5-HT)-2 receptor and insulin sensitivity: implications for atypical antipsychotics. Psychosom Med. 2005;67:748–751. doi: 10.1097/01.psy.0000174994.91245.34. [DOI] [PubMed] [Google Scholar]
- 33.Ardizzone TD, Bradley RJ, Freeman AM, Dwyer DS. Inhibition of glucose transport in PC12 cells by the atypical antipsychotics drugs risperidone and clozapine, and structural analogs of clozapine. Brain Res. 2001;923:82–90. doi: 10.1016/s0006-8993(01)03026-8. [DOI] [PubMed] [Google Scholar]
- 34.Bachelard HS, Lindsay JR. Effects of neurotropic drugs on glucose metabolism in rat brain in vivo. Biochem Pharmacol. 1966;15:1053–1058. doi: 10.1016/0006-2952(66)90270-x. [DOI] [PubMed] [Google Scholar]
- 35.Larsson S. The effect of chlorpromazine on the glucose metabolism in different parts of the goat brain. Acta Physiol Scand. 1961;53:68–74. doi: 10.1111/j.1748-1716.1961.tb02264.x. [DOI] [PubMed] [Google Scholar]
- 36.Dwyer DS, Lu X-H, Freeman AM. Neuronal glucose metabolism and schizophrenia: therapeutic prospects? Expert Rev Neurother. 2003;3:29–40. doi: 10.1002/pmic.200390005. [DOI] [PubMed] [Google Scholar]
- 37.Robinson KA, Yacoub Wasef SZ, Buse MG. At therapeutic concentrations, olanzapine does not affect basal or insulin-stimulated glucose transport in 3T3-L1 adipocytes. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:93–98. doi: 10.1016/j.pnpbp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Schaber G, Stevens I, Gaertner HJ, Dietz K, Breyer-Pfaff U. Pharmacokinetics of clozapine and its metabolites in psychiatric patients: plasma protein binding and renal clearance. Br J Clin Pharmacol. 1998;46:453–459. doi: 10.1046/j.1365-2125.1998.00822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kassahun K, Mattiuz E, Nyhart E, Jr, et al. Disposition and biotransformation of the antipsychotic agent olanzapine in humans. Drug Metab Dispos. 1997;25:81–93. [PubMed] [Google Scholar]
- 40.Wilner KD, Tensfeldt TG, Baris B, et al. Single- and multiple-dose pharmacokinetics of ziprasidone in healthy young and elderly volunteers. Br J Clin Pharmacol. 2000;49:15S–20S. doi: 10.1046/j.1365-2125.2000.00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]