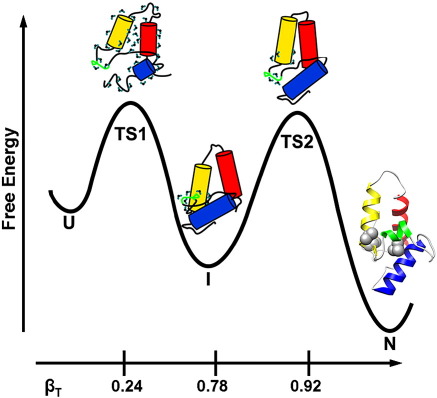
Fig. 9.
Schematic of Im7 folding. Helices are coloured as in Fig. 1. The βT value for TS1 was taken from Ref. 8; values for other states are taken from the wild-type Im7 data in Table 1. Water molecules are shown as black and green (^) symbols. While the core of TS1 is assumed to remain highly solvated,8 the data presented here demonstrate the substantial desolvation occurs as I forms. The final steps in folding involve the docking of core residues (Val42, Ile44 and L53) in the C-terminal portion of helix II and in helix III, which serve to lock the protein into its native structure. These key residues are highlighted on the ribbon diagram of native Im7 (PDB code 1AYI).22 Overpacking these positions thus prevents efficient folding to the native state such that I becomes partially or wholly populated at equilibrium.