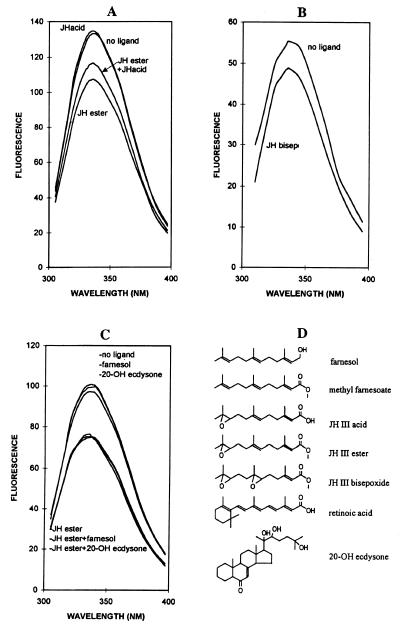
Figure 2.
Competitive binding assays show specific binding of biologically active Drosophila JHs to Drosophila USP. (A) The natural Drosophila hormone JH III ester distinctly suppressed the fluorescence of USP whereas addition of JH III acid had little effect. When incubated in combination, addition of JH acid interfered with the suppression of fluorescence by JH ester, to yield an intermediate level of suppression. This result, reproduced with independent USP preparations, indicates that JH acid competitively binds to USP, but in a manner that causes a different conformational change than caused by JH III ester. (B) The other natural Drosophila JH, JH III bisepoxide, also bound to USP in a manner that caused a distinct suppression of USP fluorescence. (C) Higher (20-OH ecdysone) and lower (farnesol) products in the terpene biosynthesis pathway than JH did not significantly bind USP, as shown by the lack of suppression of fluorescence when present alone (upper three curves) and by their lack of competitive inhibition of the suppression induced by JH III ester (lower three curves) used in the competitive binding studies. (D) Ligands used in the competitive binding studies and the related compound all-trans RA.