Abstract
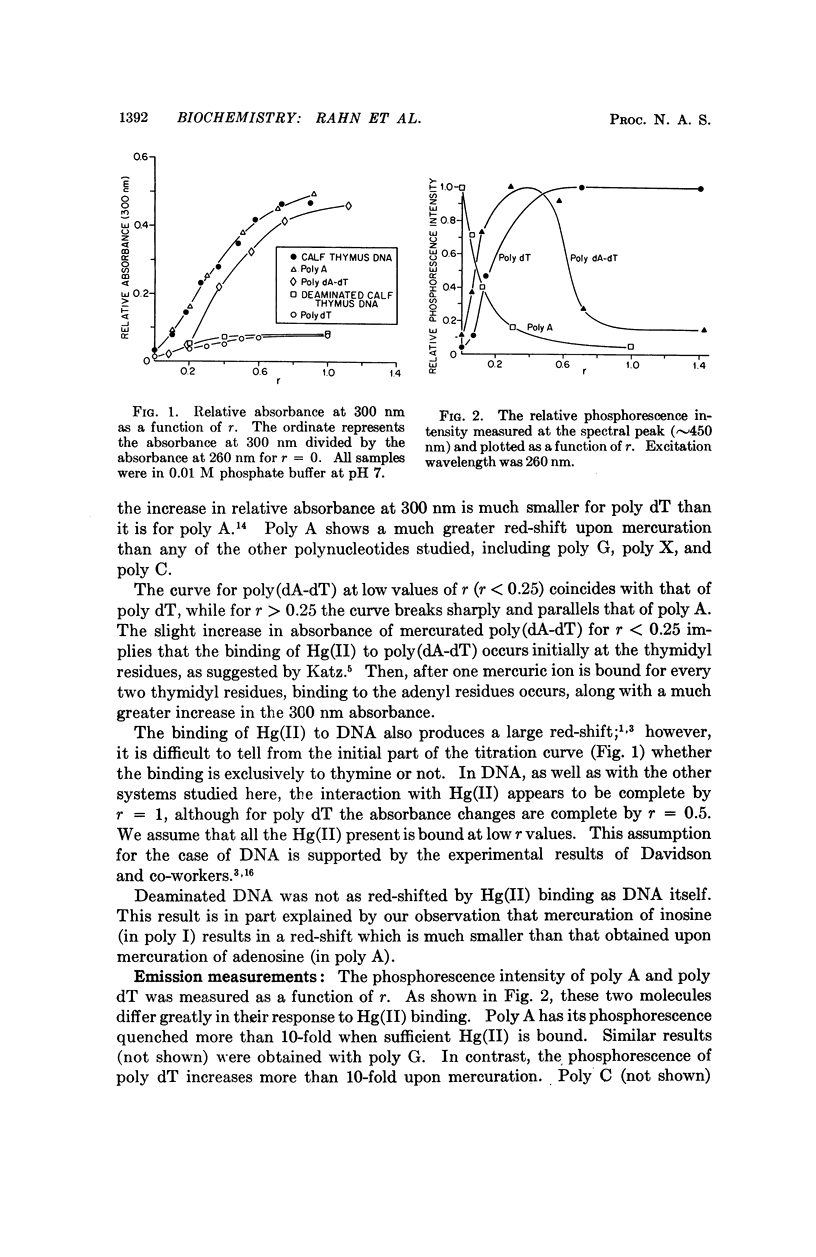
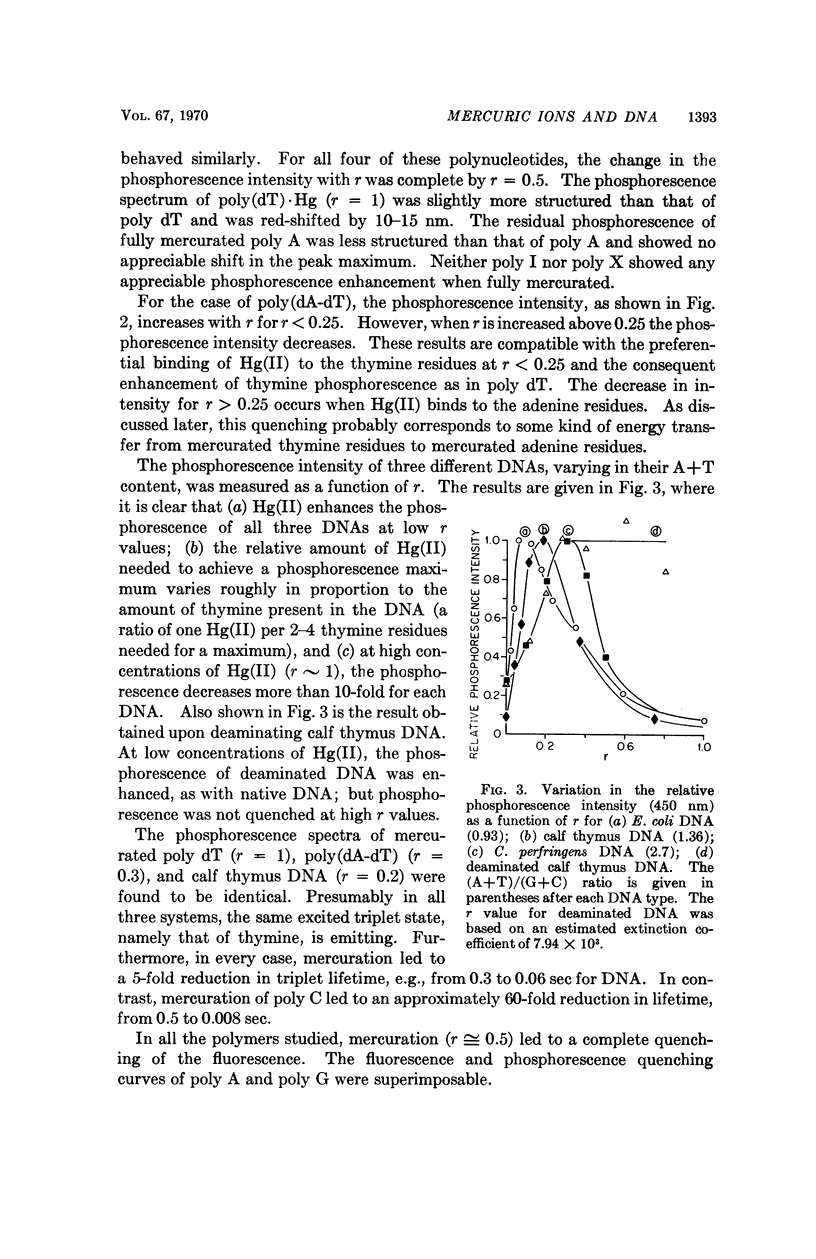
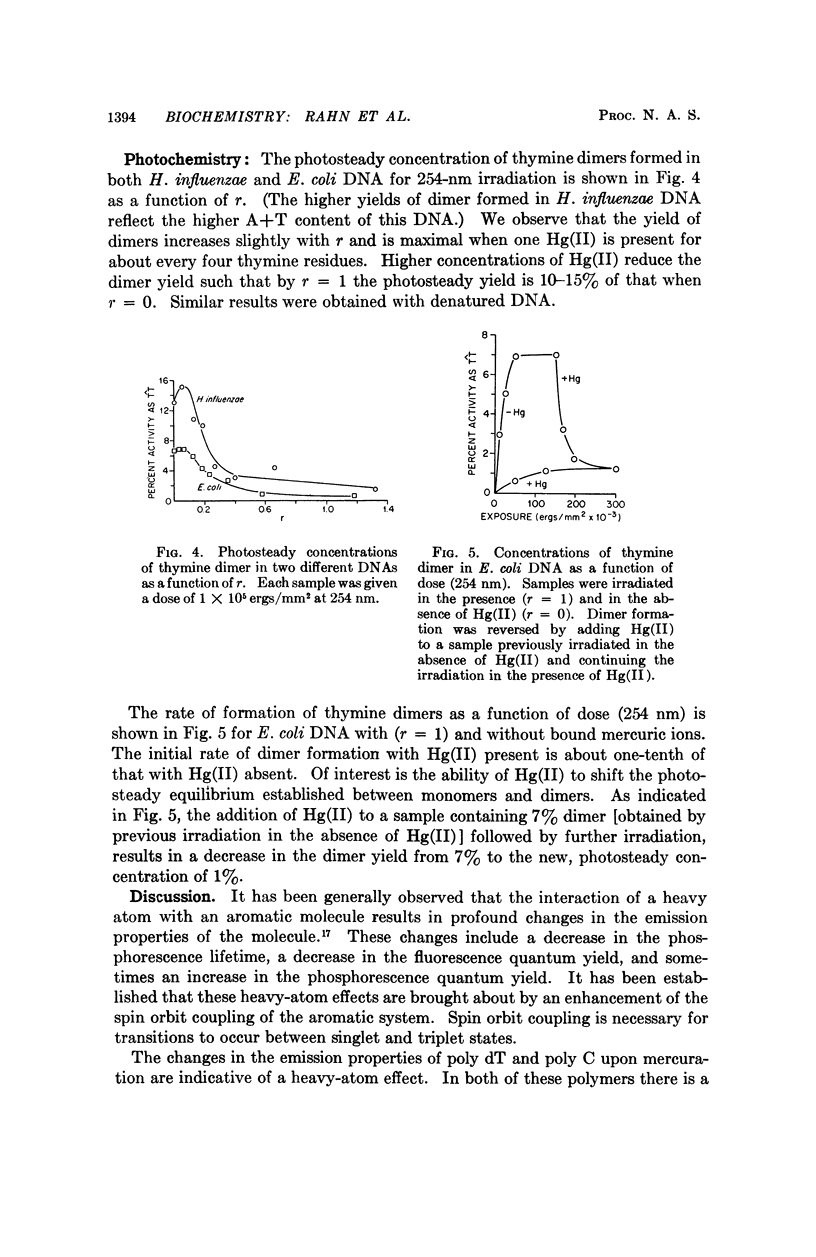
Partial mercuration of DNA, such that roughly one mercuric ion is bound for every two thymine residues, results in an enhancement of the phosphorescence by a factor of ten and a small enhancement of the photosteady thymine dimer yield. Complete mercuration of DNA [one Hg(II) added per momoner unit] results in quenching of the phosphorescence intensity and an inhibition of thymine dimer production. The enhancement of the phosphorescence is interpreted in terms of a heavy-atom effect caused by the preferential binding of Hg(II) to the thymine residues. The quenching of both the thymine phosphorescence and the rate of thymine dimerization upon complete mercuration is probably due to energy transfer from thymine to another base, presumably adenine, which when mercurated acts as an energy trap.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIDSON N., WIDHOLM J., NANDI U. S., JENSEN R., OLIVERA B. M., WANG J. C. PREPARATION AND PROPERTIES OF NATIVE CRAB DAT. Proc Natl Acad Sci U S A. 1965 Jan;53:111–118. doi: 10.1073/pnas.53.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ S. The reversible reaction of Hg (II) and double-stranded polynucleotides. A step-function theory and its significance. Biochim Biophys Acta. 1963 Feb 26;68:240–253. doi: 10.1016/0006-3002(63)90139-2. [DOI] [PubMed] [Google Scholar]
- Lamola A. A., Guéron M., Yamane T., Eisinger J., Shulman R. G. Triplet state of DNA. J Chem Phys. 1967 Oct 1;47(7):2210–2217. doi: 10.1063/1.1703293. [DOI] [PubMed] [Google Scholar]
- Longworth J. W., Rahn R. O. Energy transfer in poly-L-tyrosine as a function of the degree of ionization of the phenolic hydroxyls. I. Room-temperature fluorescence of model compounds and poly-L-tyrosine. Biochim Biophys Acta. 1967 Dec 12;147(3):526–535. doi: 10.1016/0005-2795(67)90012-8. [DOI] [PubMed] [Google Scholar]
- Millar D. B. The interaction of mercurials with helical and random polyuridylic acid. Biochim Biophys Acta. 1968 Oct 29;166(3):628–635. doi: 10.1016/0005-2787(68)90369-9. [DOI] [PubMed] [Google Scholar]
- Rahn R. O., Shulman R. G., Longworth J. W. The UV-induced triplet state in DNA. Proc Natl Acad Sci U S A. 1965 May;53(5):893–896. doi: 10.1073/pnas.53.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow R. B., Carrier W. L. Formation and destruction of pyrimidine dimers in polynucleotides by ultra-violet irradiation in the presence of proflavine. Nature. 1967 Mar 4;213(5079):906–907. doi: 10.1038/213906a0. [DOI] [PubMed] [Google Scholar]
- Setlow R. B., Carrier W. L. Pyrimidine dimers in ultraviolet-irradiated DNA's. J Mol Biol. 1966 May;17(1):237–254. doi: 10.1016/s0022-2836(66)80105-5. [DOI] [PubMed] [Google Scholar]
- Sutherland B. M., Sutherland J. C. Mechanisms of inhibition of pyrimidine dimer formation in deoxyribonucleic acid by acridine dyes. Biophys J. 1969 Mar;9(3):292–302. doi: 10.1016/S0006-3495(69)86387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMANE T., DAVIDSON N. None on the spectra of the mercury (II) and silver (I) complexes of some polyribonucleotides and ribonucleic acid. Biochim Biophys Acta. 1962 May 14;55:780–782. doi: 10.1016/0006-3002(62)90858-2. [DOI] [PubMed] [Google Scholar]
- Zmudzka B., Bollum F. J., Shugar D. Polydeoxyribouridylic acid and its complexes with polyribo- and deoxyriboadenylic acids. J Mol Biol. 1969 Nov 28;46(1):169–183. doi: 10.1016/0022-2836(69)90064-3. [DOI] [PubMed] [Google Scholar]


