Abstract
Previous reports from this laboratory described a new concept of three light reactions in plant photosynthesis comprising two short-wavelength (λ < 700 nm) photoreactions belonging to Photosystem II and one long-wavelength (λ > 700 nm) photoreaction belonging to Photosystem I. Among the electron carriers assigned to Photosystem II were cytochrome b559 and plastocyanin and to Photosystem I, cytochrome f.
According to a widely held view, the light-induced reduction of NADP by water requires the collaboration of Photosystems I and II and involves specifically cytochrome f and P700 (a portion of chlorophyll a peculiar to Photosystem I). By contrast, the new concept ascribes the light-induced reduction of NADP by water solely to the two photoreactions of Photosystem II, without the participation of Photosystem I and its components, cytochrome f and P700.
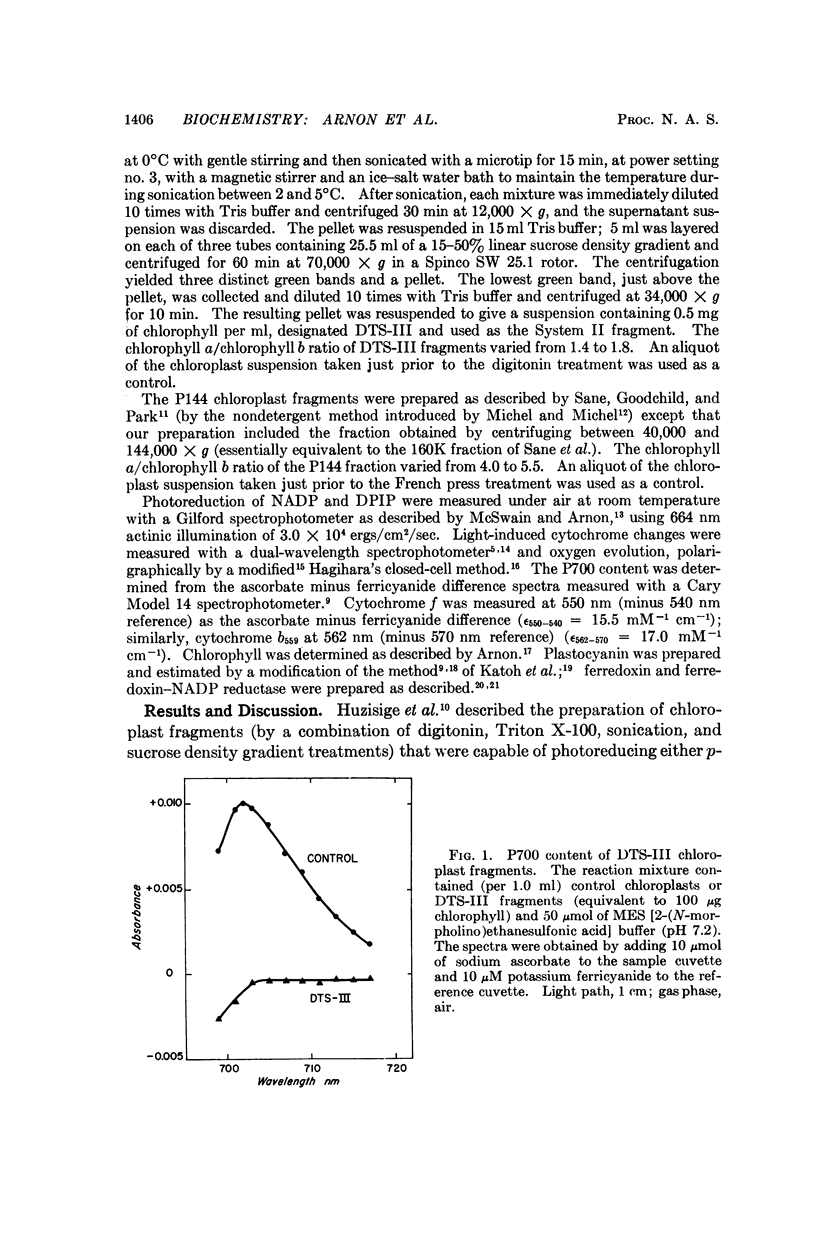
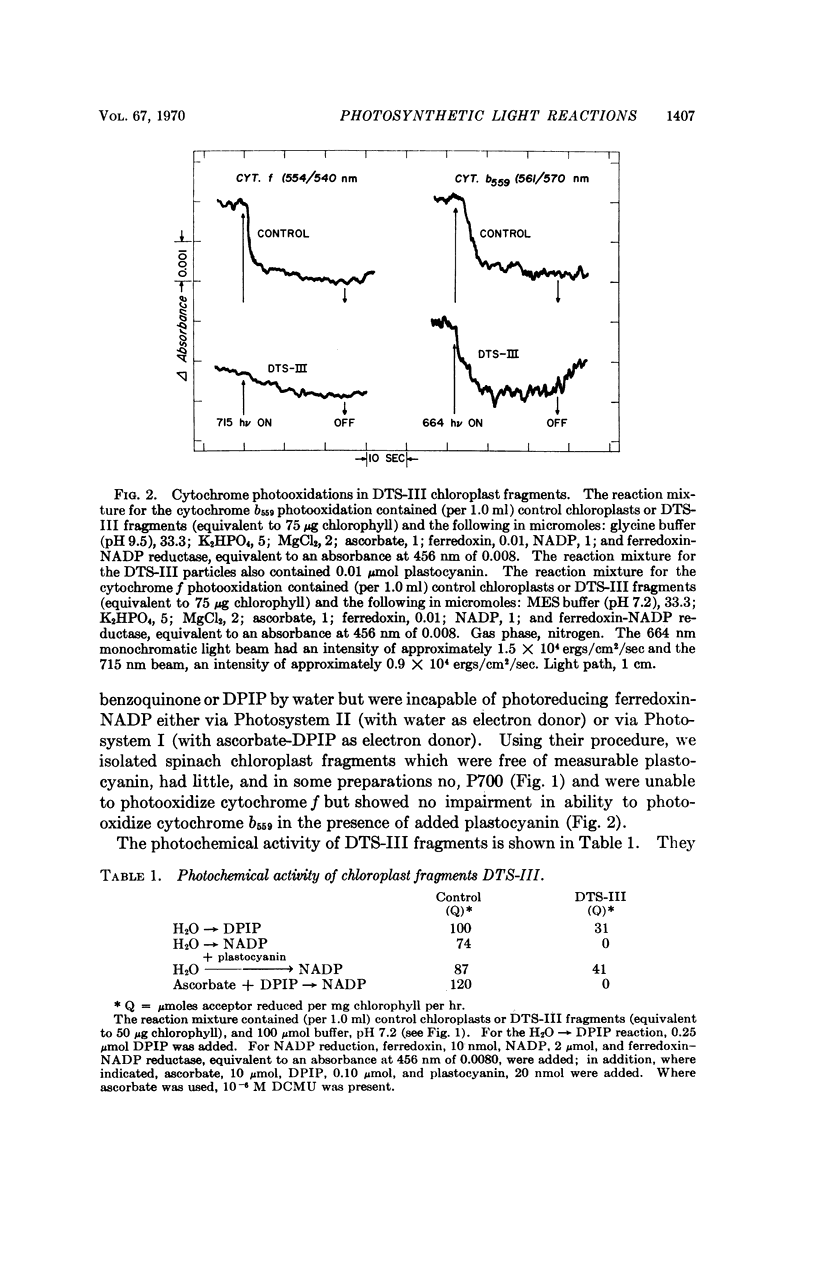
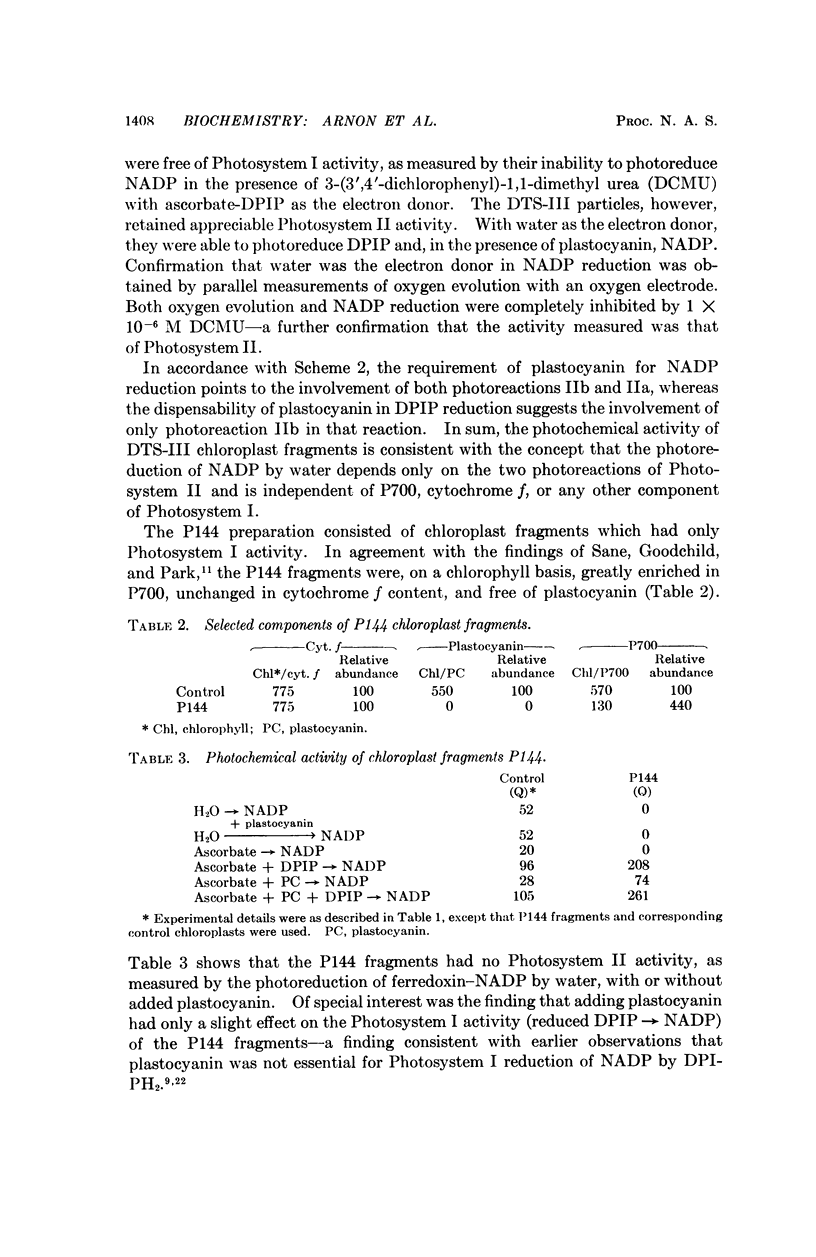
Further evidence in support of the new concept has now been obtained from chloroplast fragments. Two kinds of chloroplast fragments have been prepared: (a) one with Photosystem II activity, capable—in the presence of plastocyanin—of photoreducing NADP with water but lacking P700 and functional cytochrome f and (b) another having only Photosystem I activity, lacking plastocyanin, and enriched in P700.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARNON D. I., TSUJIMOTO H. Y., MCSWAIN B. D. ROLE OF FERREDOXIN IN PHOTOSYNTHETIC PRODUCTION OF OXYGEN AND PHOSPHORYLATION BY CHLOROPLASTS. Proc Natl Acad Sci U S A. 1964 Jun;51:1274–1282. doi: 10.1073/pnas.51.6.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. M., Boardman N. K. Fractionation of the photochemical systems of photosynthesis. I. Chlorophyll contents and photochemical activities of particles isolated from spinach chloroplasts. Bibl Laeger. 1966 Mar 14;112(3):403–421. doi: 10.1016/0926-6585(66)90244-5. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I., Tsujimoto H. Y., McSwain B. D. Ferredoxin and photosynthetic phosphorylation. Nature. 1967 May 6;214(5088):562–566. doi: 10.1038/214562a0. [DOI] [PubMed] [Google Scholar]
- Arnon D. I., Tsujimoto H. Y., McSwain B. D. Photosynthetic phosphorylation and electron transport. Nature. 1965 Sep 25;207(5004):1367–1372. doi: 10.1038/2071367a0. [DOI] [PubMed] [Google Scholar]
- Boardman N. K., Anderson J. M. Fractionation of the photochemical systems of photosynthesis. II. Cytochrome and carotenoid contents of particles isolated from spinach chloroplasts. Biochim Biophys Acta. 1967 Jul 5;143(1):187–203. doi: 10.1016/0005-2728(67)90120-x. [DOI] [PubMed] [Google Scholar]
- HAGIHARA B. Techniques for the application of polarography to mitochondrial respiration. Biochim Biophys Acta. 1961 Jan 1;46:134–142. doi: 10.1016/0006-3002(61)90656-4. [DOI] [PubMed] [Google Scholar]
- KATOH S., SHIRATORI I., TAKAMIYA A. Purification and some properties of spinach plastocyanin. J Biochem. 1962 Jan;51:32–40. doi: 10.1093/oxfordjournals.jbchem.a127497. [DOI] [PubMed] [Google Scholar]
- Knaff D. B., Arnon D. I. A concept of three light reactions in photosynthesis by green plants. Proc Natl Acad Sci U S A. 1969 Oct;64(2):715–722. doi: 10.1073/pnas.64.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaff D. B., Arnon D. I. LIGHT-INDUCED OXIDATION OF A CHLOROPLAST B-TYPE CYTOCHROME AT -189 degrees C. Proc Natl Acad Sci U S A. 1969 Jul;63(3):956–962. doi: 10.1073/pnas.63.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaff D. B., Arnon D. I. Spectral evidence for a new photoreactive component of the oxygen-evolving system in photosynthesis. Proc Natl Acad Sci U S A. 1969 Jul;63(3):963–969. doi: 10.1073/pnas.63.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSwain B. D., Arnon D. I. Enhancement effects and the identity of the two photochemical reactions of photosynthesis. Proc Natl Acad Sci U S A. 1968 Nov;61(3):989–996. doi: 10.1073/pnas.61.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sane P. V., Goodchild D. J., Park R. B. Characterization of chloroplast photosystems 1 and 2 separated by a non-detergent method. Biochim Biophys Acta. 1970 Aug 4;216(1):162–178. doi: 10.1016/0005-2728(70)90168-4. [DOI] [PubMed] [Google Scholar]


