Figure 3.
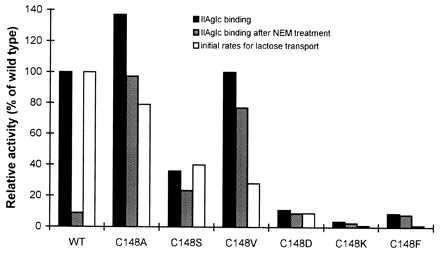
Effect of replacements of Cys-148 of lactose permease on the binding to IIAglc. Several Cys-148 replacement mutants of lac permease were tested for binding to IIAglc without (▪) or with (▨) NEM treatment (the data are corrected for IIAglc binding in the absence of TDG), and a comparison was made with the initial rate of lactose transport (□) determined previously (18). The interaction was measured as described in Fig. 2. Expression levels of lac permease from each strain were determined by quantitative phosphoimaging using polyclonal antibody against the C terminus of the permease (21) and the relative binding activity of each mutant to IIAglc was corrected for expression level using the concentration curve shown in the inset to Fig. 1 and compared with that of wild-type lac permease. Binding experiments were done at least two times and the results were reproducible.
