Figure 6. Hazard ratios for incident cardiovascular events in the JUPITER trial according to achieved concentrations of LDL cholesterol and high-sensitivity C-reactive protein (hsCRP) after initiation of rosuvastatin therapy.
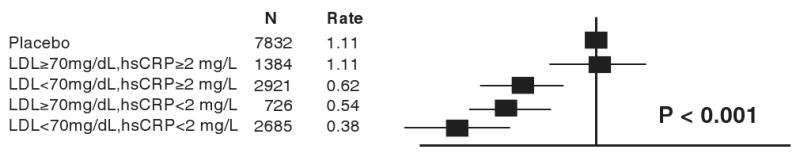
Data were adjusted for age, baseline LDL and HDL cholesterol, baseline hsCRP, blood pressure, gender, body mass index, smoking status, and parental history of premature coronary heart disease. Event rates are per 100 person-years. [Adopted from Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AM, Kastelein JJP, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ, on behalf of the JUPITER Trial Study Group. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet 2009;373:1175-1182.]
