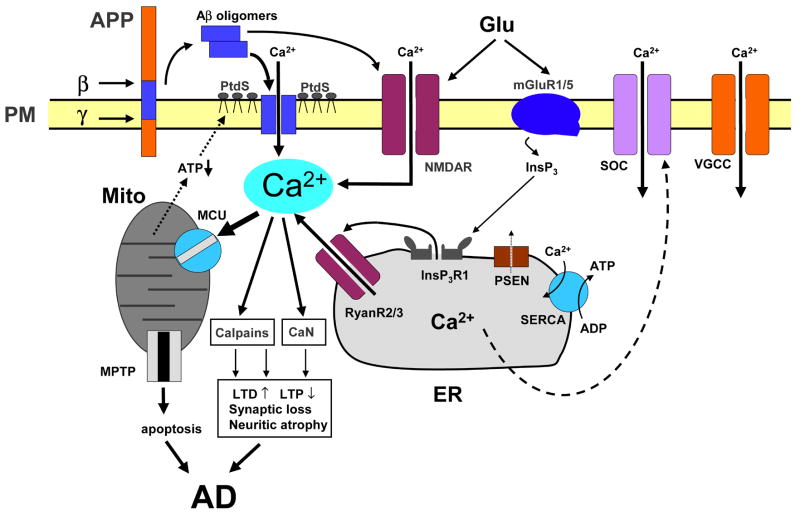
Figure 1. Calcium dysregulation in Alzheimer disease.
Sequential cleavages of β-amyloid precursor protein (APP) by β-secretase (β) and β-secretase (β) generate amyloid β peptide (Aβ). Aβ forms oligomers, which can insert into the plasma membrane and form Ca2+-permeable pores. The association of Aβ oligomers with the plasma membrane is facilitated by binding to surface phosphatidylserine (PtdS); age and Ca2+-related mitochondrial impairment leads to ATP depletion and might trigger flipping of PtdS from the inner portion of the plasma membrane to the cell surface. Reduction in ATP levels and loss of membrane integrity causes membrane depolarization, which leads to facilitation of Ca2+ influx through NMDAR and VGCC. Aβ oligomers can also affect activity of NMDAR, AMPAR and VGCC directly. Glutamate stimulates activation of mGluR1/5 receptors, production of InsP3 and InsP3R -mediated Ca2+ release from the ER. Presenilins (PS) function as an ER Ca2+-leak channels and many FAD mutations impair Ca2+-leak-channel function of PS, resulting in excessive accumulation of Ca2+ in the ER. Increased ER Ca2+ levels result in enhanced Ca2+ release through InsP3 -gated InsP3R 1 and Ca2+-gated RyanR(2/3). PS might also modulate activity of InsP3R, RyanR and SERCA pump directly. The activity of store-operated Ca2+ channels on the plasma membrane can be affected indirectly by PS mutations through the modulation of SERCA activity. Elevated cytosolic Ca2+ levels result in the activation of calcineurin (CaN) and calpains and lead to facilitation of LTD, inhibition of LTP, modification of neuronal cytoskeleton, synaptic loss and neuritic atrophy. Excessive Ca2+ is taken up by mitochondria through mitochondrial Ca2+ uniporter (MCU), eventually leading to opening of mitochondrial permeability-transition pore (mtPTP) and apoptosis.