Fig. 1.
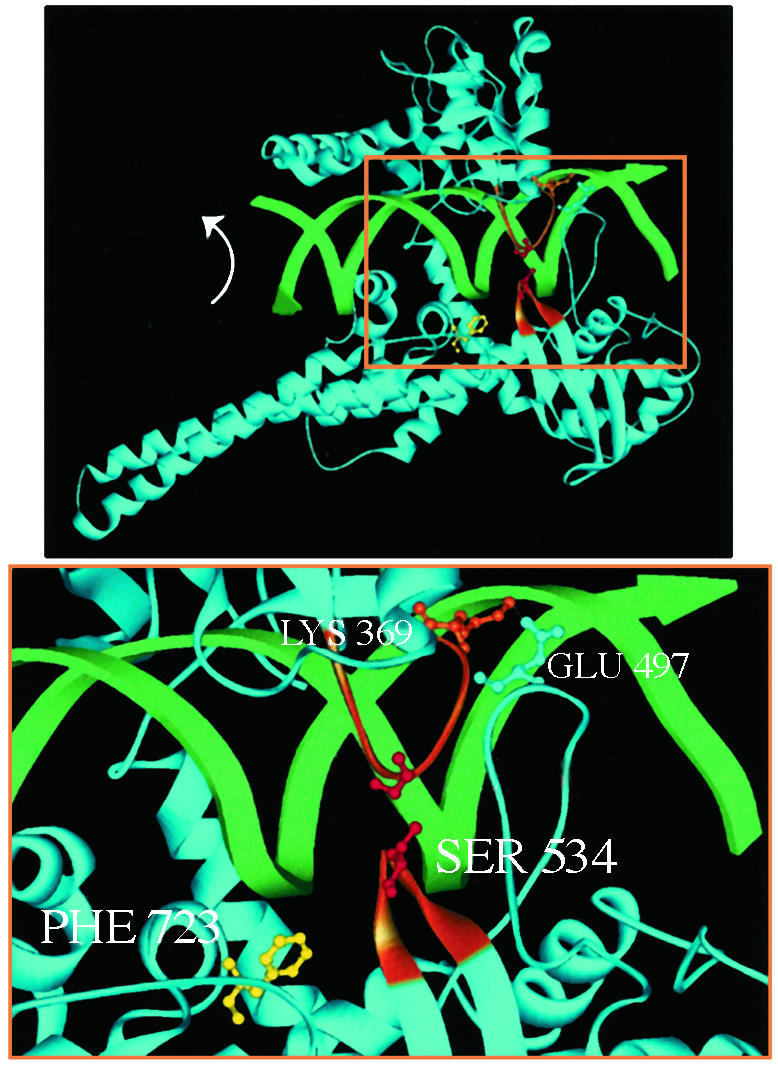
(Upper) The bilobed structure of Top1 clamped around duplex DNA. Two α-helices extend from the protein clamp to link the core with the active-site tyrosine domain. The active-site tyrosine (shown here as the Phe-723 mutant, yellow) is poised to cleave the scissile DNA strand. DNA rotation in the covalent complex is indicated (arrow). (Lower) The higher magnification of the boxed area in Upper highlights the lip domains that close the protein clamp. Two loops constitute the lower lip: one loop contains Ser-534 (orange), and the other loop contains Glu-497, which forms a salt bridge with Lys-369 in the upper lip (orange). Cysteine substitutions for Gly-365 and Ser-534 that are predicted to form a disulfide bond are shown in red.
