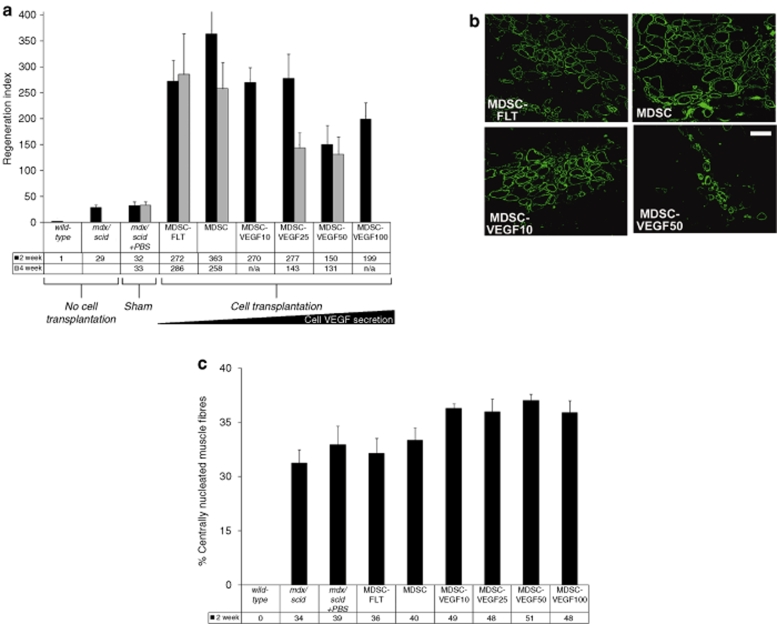
Figure 3.
In vivo MDSC transplantation to skeletal muscle of dystrophic tissue; restoration of dystrophin. (a) We used the RI (RI = number of dystrophin-positive fibers per 105 donor cells) to compare the regeneration efficiency of the different populations. We quantified the total number of dystrophin-positive fibers after transplantations of MDSCs, MDSC-FLT1 MDSC-VEGF10, MDSC-VEGF25 and MDSC-VEGF50, and MDSC-VEGF100 populations, N = 6–16. Transplantation of the MDSC-VEGF50 and MDSC-VEGF100 populations resulted in significantly reduced numbers of dystropin-positive myofibers as compared to the MDSC control. (b) Dystrophin immunostaining in the tissue cross-sections of gastrocnemius muscles of mdx mice 2 weeks post-transplantation revealed new dystrophin-positive myofibers (green) within the dystrophic animals that lack dystrophin expression (original magnification ×200, Bar = 100 µm). (c) In vivo examination of endogenous muscle regeneration of mdx tissue within 600 µm of the engraftment area of the donor cells. When injected into 8–10-week old mdx/scid mice, both MDSC-VEGF10 and MDSC-VEGF25 revealed significantly higher rates of centro-nucleated muscle fibers independent of the donor MDSCs, as compared to noninjected mdx/scid mice. While there was no evidence of a decrease in centro-nucleation between noninjured mice and the MDSC-FLT group, there was a significant difference between the disparity between the noninjected mice and VEGF10. MDSC, muscle-derived stem cell; RI, regeneration index; VEGF, vascular endothelial growth factor.