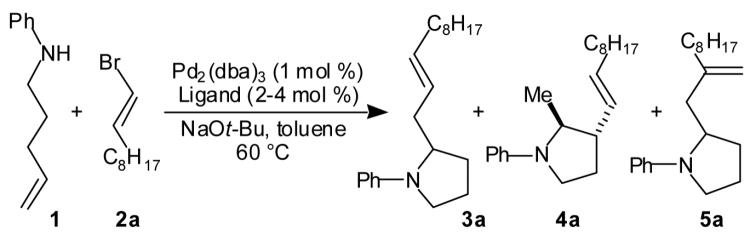
Table 1.
Catalyst optimization.[a]
| ||||||
|---|---|---|---|---|---|---|
| Entry | Ligand | 3a/4a[b] | 3a/5a[b] | Isolated yield[c] | ||
| 1 | dppb | 20:1 | 3:1 | 60 | ||
| 2 | dppe[d] | 15:1 | 5:1 | |||
| 3 | PCy3[d] | 13:1 | 4:1 | |||
| 4 | P(2-furyl)3 | 21:1 | 19:1 | 81 | ||
Conditions: amine 1 (1.0 equiv), vinyl bromide 2a (1.1 equiv), NaOt-Bu (1.2 equiv), Pd2(dba)3 (1 mol%), Ligand (2 mol% of bidentate ligand or 4 mol% of monodentate ligand), toluene (0.25 M), 60 °C.
Ratios determined by GC analysis of crude reaction mixtures.
Isolated yields refer to the total yield of an inseparable mixture of products 3a, 4a, and 5a.
This reaction was conducted at 110 °C.