Table 3.
Stereoselective synthesis of disubstituted N-aryl-2-allylpyrroldines.[a]
| Entry | Amine | Vinyl bromide | Product (E/Z ratio)[b] | Regio[b] | Yield [%][b] |
|---|---|---|---|---|---|
| 1 |
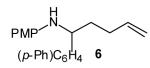
|
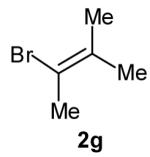
|
|
>20:1 | 72 (dr 7:1) |
| 2 |
|
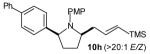
|
20:1 | 82 (dr >20:1) |
|
| 3 |
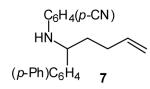
|
|
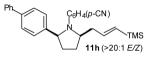
|
>20:1 | 71 (dr >20:1) |
| 4 |
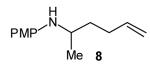
|
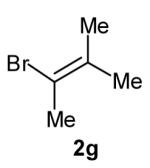
|
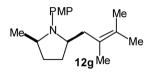
|
>20:1 | 84 (dr >20:1) |
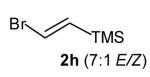
| 5 |
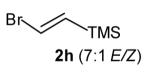
|
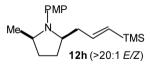
|
7:1 | 79 (dr >20:1) |
|
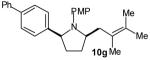
| 6 |
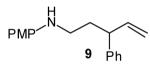
|
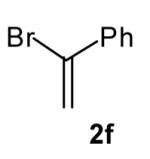
|
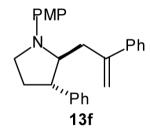
|
>20:1 | 55 (dr 10:1) |
| 7 |
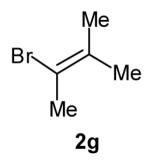
|
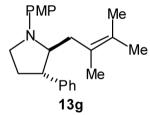
|
>20:1 | 64[c] (dr 10:1) |
|
| 8 |
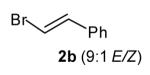
|

|
3.6:1 | 61[c] (dr >20:1) |
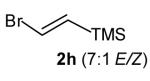
Conditions: amine (1.0 equiv), vinyl bromide 2 (1.4–2.0 equiv), NaOt-Bu (1.2 equiv), Pd2(dba)3 (1 mol %), P(2-furyl)3 (4 mol%), toluene (0.25 M ), 110 °C.
Product ratios refer to the isolated material, as judged by 1H NMR analysis. Product ratios and yields refer to the average of two or more experiments.
Dppe (2 mol %) used as ligand in this reaction. PMP = p-methoxyphenyl.
