Table 4.
Synthesis of 2-allyl pyrrolidines and indolines via tandem reactions of primary amines.[a]
| Entry | Amine | Aryl bromide | Vinyl bromide | Product (E/Z ratio)[b] | Yield [%][b] |
|---|---|---|---|---|---|
| 1 |
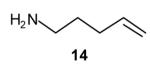
|
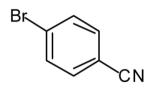
|
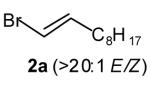
|
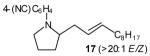
|
65[c],[e],[f] |
| 2 |
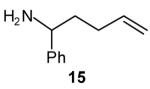
|
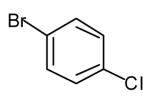
|
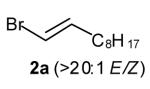
|
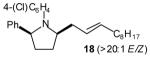
|
50[c],[e],[f] (>20:1 dr) |
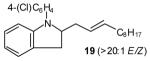
| 3 |
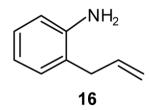
|

|
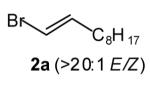
|
|
60[d],[f] |
| 4 |
|
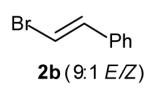
|
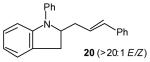
|
69[d] |
Conditions: amine (1.0 equiv), aryl bromide (1.0 equiv), NaOt-Bu (2.4 equiv), Pd2(dba)3 (1 mol%), 2-di-t-butylphosphinobiphenyl(2 mol%), toluene (0.25 M), 60–80 °C, then dppe or DPEphos (2 mol%) and vinyl bromide (1.2 equiv), 105–110 °C.
Product ratios refer to the isolated material, as judged by 1H NMR analysis. Product ratios and yields refer to the average of two or more experiments.
This reaction was conducted with dppe as the second ligand.
This reaction was conducted with DPEphos as the second ligand.
This material contained ~3–10 % of an inseparable regioisomer analogous to 4a.
This material contained ~7–10 % of an inseparable impurity analogous to 5a.
