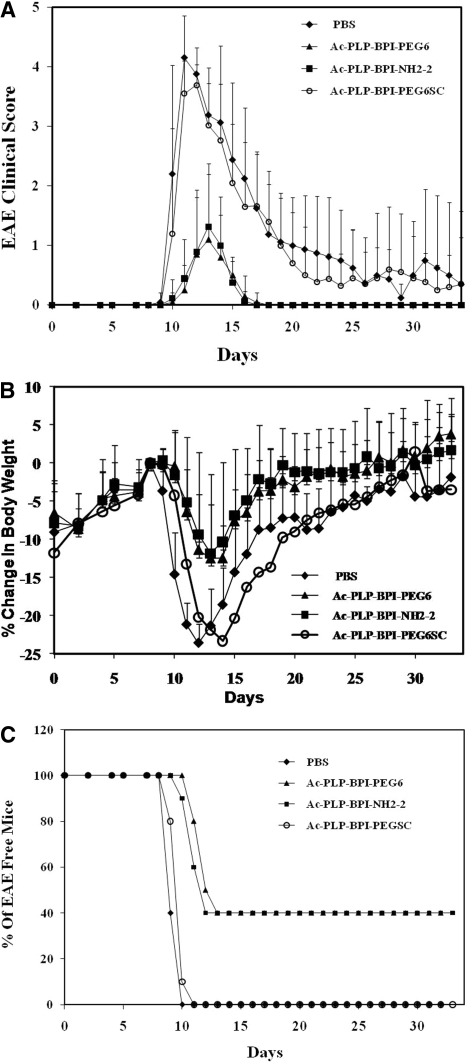
Fig. 2.
In vivo EAE-suppressive activity of BPI derivatives in the mouse EAE model. PLP139-151/CFA-immunized mice received intravenous injections of the indicated peptide (100 nmol/mouse/day) on days 4 and 7. A, clinical EAE disease score. B, change in body weight. C, incidence of disease. Results are expressed as the mean ± S.D. (n = 10). There are significant differences between BPI-treated and PBS-treated groups in clinical disease scores and loss of body weight: Ac-PLP-BPI-NH2-2, p < 0.001 and Ac-PLP-BPI-EG6, p < 0.001. There was no significant difference (p > 0.05) in EAE clinical scores and loss of body weight between scrambled peptide (Ac-PLP-BPI-PEGSC) and PBS groups. In addition, no significant difference (p > 0.05) between Ac-PLP-BPI-NH2-2 and Ac-PLP-BPI-PEG6 was observed.