Figure 1. Evaluation of apoptosis, Fas, FasL and CD45 expression in Jurkat cells treated with GalXM.
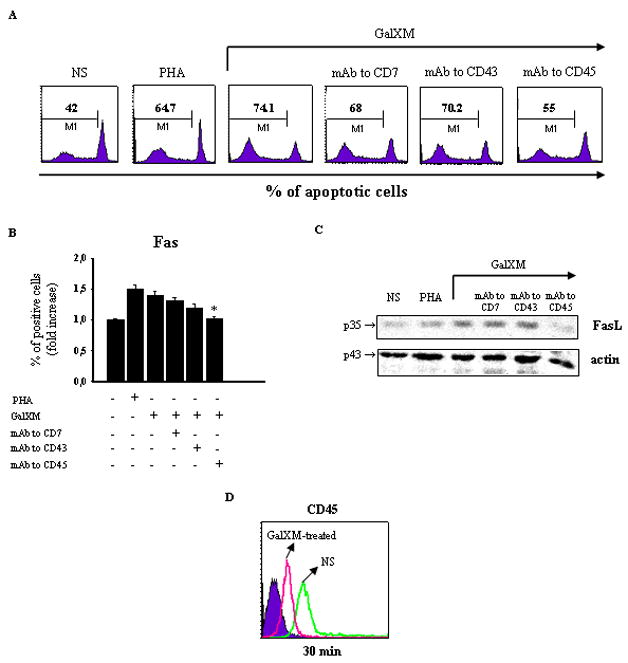
Jurkat cells (1 × 106/ml) were incubated in the presence or absence (NS) of mAbs to CD7, CD43 (both 0.5 μg/ml), or CD45 (1 μg/ml) for 30 min at 4°C and then cultured for 18 h at 37°C and 5% CO2 in the presence or absence of PHA or GalXM (both 10 μg/ml). The percentage of Jurkat cells undergoing apoptosis was evaluated by PI staining and analyzed using a FACScan flow cytofluorometer (A). Data are expressed as percentage of apoptotic cells of one representative experiment out of ten experiments with similar results. For evaluation of Fas expression, Jurkat cells (1 × 106), treated as above described, were reacted with FITC-labeled mAb to CD95 (Fas) and analyzed by flow cytometry (B). Data are expressed as the fold increase of the percentage of positive cells. For evaluation of FasL protein level, Jurkat cells (5 × 106/ml), treated as above described, were lysated and subjected to western blot. Membranes were incubated with antibody to FasL (C). The specificity of mAbs to CD7, CD43 and CD45 was testified by the inefficacy of isotype controls. * p < 0.05 mAb to CD45 plus GalXM-treated cells vs GalXM-treated cells. Jurkat cells (1 × 106/ml) were incubated in the presence or absence (NS) of GalXM (10 μg/ml) for 30 min at 37°C and 5% CO2. After incubation, cells were reacted with RPE-labeled mAb to CD45 and analyzed by flow cytometry. FACScan histogram of mean of fluorescence intensity (MFI) of CD45 expression of one representative experiment out of five with similar results was reported (D). Controls staining of cells with irrelevant antibodies were used to obtain background fluorescence values.
