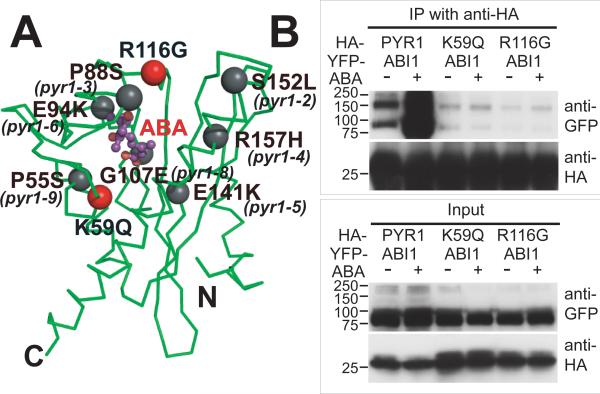
Fig. 3.
Disruption of PYR1-ABI1 interactions by single-site PYR1 mutations. (A) PYR1 mutants designed from the structure (red) and identified after chemical mutagenesis and screening (gray) (6) are mapped to the PYR1 subunit structure (green Cα trace). (B) Co-immunoprecipitation from extracts of plant leaves expressing YFP-tagged ABI1 phosphatase with HA-tagged wild-type and mutant PYR1 proteins in planta, both in the absence (−) and presence (+) of exogenously applied ABA. After co-immunoprecipitation by an anti-HA matrix, immunoprecipitated (top) and input (bottom) samples were detected with anti GFP and anti-HA antibodies (labeled at right). Structure-based PYR1 mutants designed to disrupt ABA binding (K59Q and R116G) folded properly (fig. S5), but lost ABA-induced interactions with ABI1 phosphatase.