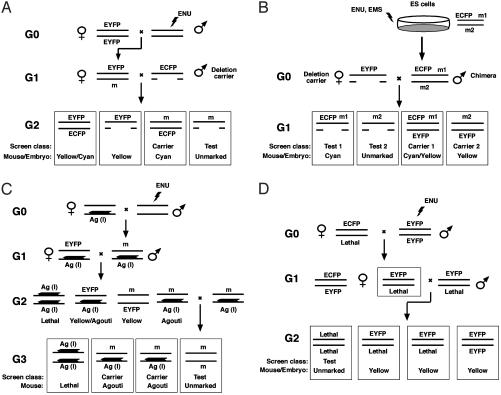
Fig. 4.
Marking directed mutagenesis screens using the pCX-YNC reporter. (A) A traditional hemizygosity screen using visibly marked chromosomes and deletions. In this example an ENU mutagenized male (G0) is mated with an EYFP homozygous female to generate an EYFP marked G1 (G1) female heterozygous for an ENU-induced mutation (m). Mutations in the region are uncovered in a test cross with an ECFP marked deletion carrier male. The G2 (G2) test class progeny are color-coded to visually distinguish the test class (unmarked), mutation carrier (cyan), and two discard classes (yellow/cyan and yellow). (B) A hemizygosity screen combining visibly marked chromosomes, deletions, and ES cell mutagenesis. ENU or EMS mutagenized ES cell clones are used to produce germ-line competent chimeric mice. Mutations in the region produced in the diploid ES cells residing on the ECFP marked chromosome (m1) and on the unmarked chromosome (m2) are uncovered in a single-generation test cross with an EYFP marked deletion carrier female. The G1 test class progeny are color-coded to visually distinguish the m1 test class (cyan), the m1 carrier (cyan/yellow), the m2 test class (unmarked), and the m2 carrier (yellow). An EGFP-marked C57BL/6J host blastocyst can be used to ensure that offspring are ES cell-derived. Host-derived embryos and mice are green and can be distinguished from the cyan and unmarked ES cell-derived test class. Although yellow and green fluorescence are not readily distinguished, the m2 carrier can be identified based on 129-derived ES cell agouti coat color. (C) A regional homozygosity screen using a visibly marked, recessive lethal inversion. An ENU-treated G0 male is mated with a marked inversion-heterozygote female. CRE-loxP mediated chromosome engineering has been used to produce recessive lethal inversion chromosomes dominantly marked with the K14-Agouti transgene (Ag). The mutation (m) carrying marked inversion-heterozygote G1 offpsring are mated with a marked inversion-heterozygote that is also EYFP- (or ECFP)-marked to produce G2 progeny. The mutation carrying marked inversion-heterozygous G2 mice are selected based on the absence of yellow fluorescence. These G2 offspring can be intercrossed to produce the G3 test class progeny that are marked to distinguish the homozygous mutation test class (unmarked) and carrier (e.g., agouti). Homozygosity for the marked inversion is lethal (l), typically owing to the disruption of an essential locus at an inversion breakpoint. (D) A genomewide dominant suppressor screen using visibly marked chromosomes. In this example an ENU-treated EYFP homozygous G0 male is mated with an ECFP marked female heterozygous for the sensitizing recessive mutation (e.g., embryonic lethality). In this two-generation scheme, G1 females heterozygous for the lethal mutation that are also heterozygous for a genomewide complement of ENU-induced mutations are selected based on yellow fluorescence and mated with an EYFP marked male that is heterozygous for the sensitizing lethal mutation to produce the G2 test cross progeny. Typically, the unmarked class homozygous for the sensitizing recessive lethal mutation will be absent in the G2 litter. The recovery of unmarked G2 progeny reveals an ENU-induced mutation that acts as a dominant suppressor of the lethal phenotype.