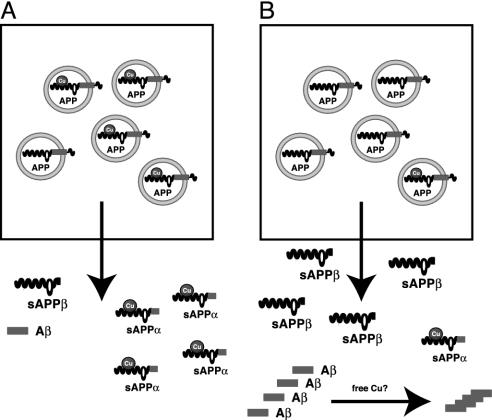
Fig. 6.
Model for the metallobiology of APP. APP is involved in Cu homeostasis in brain with implications for AD. (A) Normal interaction of APP with Cu. Cu binds to the N-terminal domain of APP that is in the lumen of intracellular compartments. After fusion with the plasma membrane, Cu is released into the extracellular space as α-secretory APP–Cu complexes. In this case, APP-Cu is cleaved by the α-secretase close to the plasma membrane and then released. APP molecules without Cu are cleaved by β- and γ-secretase activities within intracellular compartments, which is probably due to a different conformation as compared with APP charged with Cu. Low levels of Aβ peptides are secreted from the cell. (B) Depletion of Cu, as observed in brains of AD patients and APP transgenic mice, enhances amyloidogenic processing of APP. Our data show reduced SOD-1 activity and Cu levels in brain tissue of APP transgenic mice that could both be rescued by Cu treatment. This is a coherent finding, because intracellular Cu availability recently turned out to be a critical determinant of SOD-1 activity (45). An enhanced release of Aβ peptides might act as a trap for Cu, although we did not find evidence for this notion in our in vivo studies.