Figure 1.
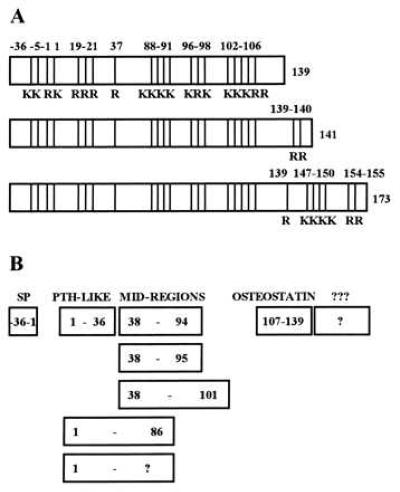
(A) Structural maps of the three PTHrP initial translation products. “K” indicates lysine and “R” arginine. The three translation products are identical in their signal peptide (−36 to −1) region and in their remaining coding regions from amino acids 1 through 139. Alternative splicing gives rise to two C-terminally extended versions of the peptide with lengths of 141 and 173 amino acids. Note the multibasic clusters: the KKXRK at −5 to −1, the single R at +37, and the KR clusters in the 88–106 region are known to be prohormone convertase substrate sites. (B) The mature secretory forms of PTHrP. After cleavage of the signal and pro-peptide in the −36 to −1 region, the secretory forms of PTHrP include PTHrP(1–36) which interacts with the PTH/PTHrP receptor, three mid-region species, and at least two C-terminal species.
