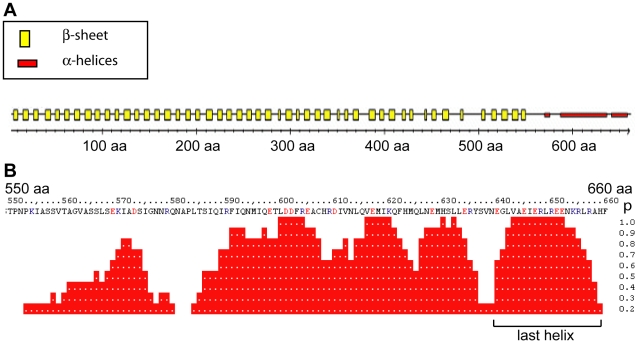
Figure 2. NEDD1 interacts with γ-tubulin through a helical region.
PredictProtein (www.predictprotein.org) was used to assess the secondary structure of human NEDD1. (A) The majority of NEDD1 protein is predicted to be composed of β-sheets, spanning amino acids 1–550. However, the region of NEDD1 between amino acids 550–660 is predicted to be predominantly α-helical. (B) Closer analysis of the α-helical structure for residues 550–660 reveals that this region is predicted to encode three regions with a high probability (p) of being helical. The last helix (640–660 aa) has a particularly high probability (p) of being helical.