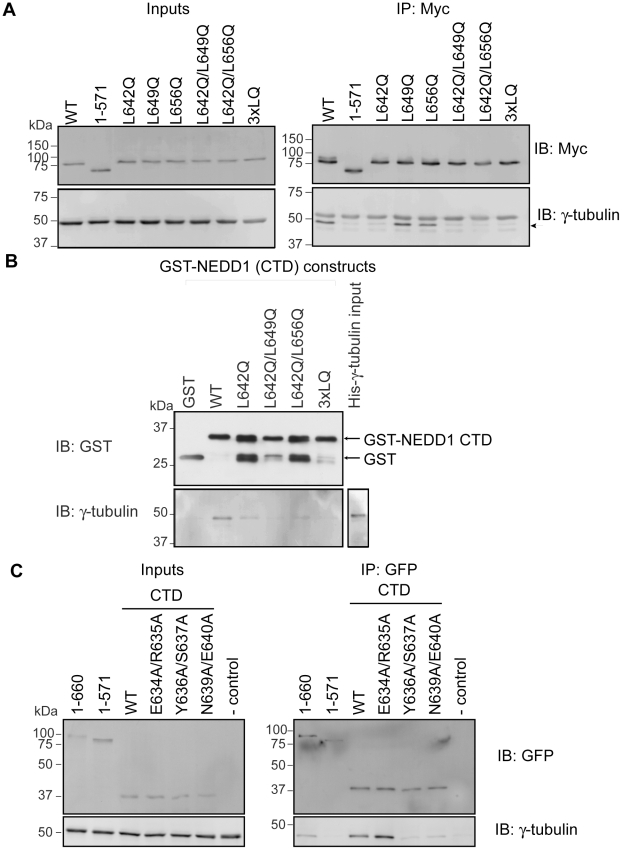
Figure 5. Specific mutations within NEDD1 show reduced binding to γ-tubulin.
(A) Mutations were introduced into a Myc-tagged full length NEDD1 construct and transfected into HEK293T cells. The expression of all NEDD1 constructs and γ-tubulin is confirmed in the inputs (1/20 lysates loaded). γ-tubulin is immunoprecipitated with wild type (WT) full length NEDD1 but not with NEDD1 (1–571 aa). There is a loss of γ-tubulin immunoprecipitated with the L642Q mutant NEDD1, but not with the single NEDD1 mutants of L649Q or L656Q. Double mutants including L642Q also reduce the immunoprecipitation of γ-tubulin, as does the L642Q/L649Q/L656Q (3xLQ) triple mutant. The upper band in the γ-tubulin immunoblot is IgG. (B) The interaction of WT or mutant NEDD1 CTD with γ-tubulin was assessed in vitro. Recombinant GST or GST-NEDD1 CTD mutants bound to glutathione sepharose beads were incubated with His-γ-tubulin. All GST-tagged proteins are expressed well and bind to the beads, and His-γ-tubulin is also expressed. After incubation with the beads and removal of unbound proteins, γ-tubulin is not bound to GST alone, but is bound to WT GST-NEDD1 CTD. There is reduced binding of γ-tubulin to L642Q NEDD1, and to the double and triple mutants. The lower bands in the GST-NEDD1 CTD mutant protein lanes are likely to represent cleaved GST. (C) Selected mutations were introduced into a GFP-tagged NEDD1 CTD construct and transfected into HEK293T cells. Expression of all NEDD1 constructs and γ-tubulin is confirmed in the inputs (1/20 lysates loaded). γ-tubulin is immunoprecipitated with full length (1–660 aa) NEDD1 but not with NEDD1 (1–571 aa). WT NEDD1 CTD is able to immunoprecipitate γ-tubulin, as seen previously, as is the E634A/R635A mutant. There is a loss of γ-tubulin immunoprecipitated with the Y636A/S637A and N639A/E640A mutant constructs of NEDD1. The lane indicating - control has no antibody added.