Summary
Objective
Our objective was to compare the physiochemical properties and erosion potentials between beverages available in the UK and the US.
Methods
The physiochemical properties (pH, titratable acidity and fluoride concentration) and erosion potential on enamel surfaces of beverages available in the UK were compared to similar beverages from the US. Enamel windows were exposed to beverages for 25 hours. Teeth were sectioned through the windows, and lesion depths were defined as the average distance between the original tooth structure and the base of demineralization.
Results
The pH was lower in UK apple juice, orange juice, Diet Pepsi® and Sprite Zero® (p<0.05), and higher in UK orange soda and diet orange soda than in similar US beverages (p<0.05). Titratable acidities were higher in UK apple juice, orange juice, orange soda, diet orange soda and Sprite® (p<0.01), and lower in UK Sunny D® than in the US counterpart (p<0.001). Fluoride concentrations were lower in UK apple juice, orange juice, Coke®, and Diet Coke®, Sprite® and Sprite Zero® (p<0.001), and higher in UK orange soda, diet orange soda, Pepsi® and Diet Pepsi® than in their US counterparts (p<0.001). Lesion depths were higher in UK apple juice, orange juice, Diet Coke®, Sprite® and Sprite Zero® than in their US counterparts (p<0.05). Lesion depths were associated with pH (p=0.010) and country of origin (p=0.002).
Conclusions
Under similar laboratory conditions, the physiochemical properties and erosion potentials on enamel surfaces differed between some, but not all, beverages available in the UK and the US.
Keywords: Beverages, pH, titratable acidity, erosion, fluoride
Introduction
Dental erosion is defined as the pathologic, chronic loss of enamel and/or dentine resulting from chemical removal of the tooth surface, excluding tooth loss associated with bacterial produced acid.1,2
The literature suggests that different prevalence rates of erosion exist between Europe and the United States (US). Bartlett et al reported that studies conducted in Europe have cited erosion as being the most common and destructive form of tooth wear.3 The authors noted that studies conducted in North America have focused on attrition, rather than tooth wear and erosion.3 Dental erosion is an oral health concern in the United Kingdom (UK), yet erosion is not reported as frequently in the US.
Studies conducted in European countries suggest that the incidence of dental erosion ranges from 5 to 60%.4–9 Dugmore and Rock reported a tooth erosion prevalence of 59.7% in a random sample of 12-year-old British children from Leicestershire and Rutland counties participating in a National Dental Health Survey.5 El Aidi et al reported a baseline erosion prevalence of 32.2%, which increased to 42.8% during 1.5 years, in a convenience sample of 12-year-olds from the central Netherlands.6 Similar results were reported for 5-year-old Irish school children with 47% exhibiting some erosion.7 Deery et al. reported similar rates of erosion in adolescents from the UK and the US in a convenience sample, and acknowledged that limited data are available on the prevalence of erosion in the US, particularly in young children.10 Mathew et al reported 36.5% of university athletes from the Midwest (US) had erosion.11 More recently, McGuire et al reported that 45.9% of US adolescents participating in the 2003–04 National Health and Nutrition Examination Survey had evidence of erosion on at least 1 tooth.12 The limited literature combined with limited interest by the US dental community supports the hypothesis that erosion is less problematic in the US than in the UK.
Numerous studies have investigated the role of diet in the etiology of erosion.1,2 Acidic compounds such as citric, malic and phosphoric acids added to or found naturally in beverages and foods increase the erosion potential of that particular food.2 The presence of phosphate, calcium and fluoride can reduce the erosion potential of a beverage in vitro.13–18 The quantity of fluoride typically found in soft drinks offers little protection, but higher concentrations of fluoride in such beverages could reduce the erosion potential.13
The pH, titratable acidities and in vitro erosion potentials of beverages available in Europe have been studied extensively,13–16,18–21 while investigation of beverages available in the US is more limited.17,22,23 Most investigators have reported that 100% juices, carbonated beverages (i.e., pop, soda), sports drinks and energy drinks from both Europe and the US are potentially erosive, and that calcium addition or fortification minimizes the erosion potential. However, the erosive potential of beverages available in Europe cannot be directly compared to the erosion potential of those available in the US because the research was conducted by different investigators using different techniques in different laboratories at different time points.
Thus, differences in erosion observed between Europe and US could be due to different beverage erosion potentials and/or different drinking habits. We hypothesize that beverages commonly consumed in the UK have higher erosion potentials than those in the US. The objective of this study was to compare the physiochemical properties and erosion potentials on enamel surfaces between beverages available in the UK and the US.
Materials and Methods
Experimental design
An in vitro design was used to compare physiochemical properties (pH, titratable acidity and fluoride concentration) and erosion potentials on enamel surfaces of beverages available in the UK to those available in the US.
Beverage selection
Commonly consumed, potentially erosive, ready-to-drink beverages were identified in the UK and US. Examples of commonly consumed UK beverages were matched to similar US beverages, while examples of commonly consumed US beverages were matched to similar UK beverages for a total of 11 beverage pairs. UK beverages were purchased in the UK and shipped to Iowa; US beverages were purchased in Iowa. Beverages were stored at room temperature, or refrigerated according to manufacturer’s recommendations prior to analyses. Beverages included apple juice (UK: Del Monte®; US: Minute Maid®), orange juice (UK: Del Monte®; US: Minute Maid®) Coke®, Diet Coke®, orange soda (UK: Tango®; US: Fanta), diet orange soda (UK: Diet Tango®; US: Diet Fanta®), Pepsi®, Diet Pepsi®, Sprite®, Sprite Zero® and Sunny D®.
Physiochemical properties
The pH and titratable acidities of each beverage were measured in triplicate using an automatic titrator (Metrohm E512 analog pH meter, Brinkmann Instruments, Inc, Westbury, NY 1982).17 The titratable acidity was measured by adding 1M KOH to 50 ml beverage until the pH reached 7.0. Fluoride concentrations were read directly using an ion-specific electrode (model 96-09-00; Orion Research Inc, Cambridge, MA, 1983).
Tooth preparation
Extracted caries-free molars and premolars were selected from a pooled supply, disinfected using fixative and cleaned of soft tissue and debris.17 Soft tissue debris was removed using a razor blade, tweezers, sonicator (Branson 1510, Branson Ultrasonics Company, Danbury, CT) and tooth brush. Teeth were painted with fingernail polish to isolate one 1×4 mm window of enamel on a flat, smooth surface. Each tooth served as an independent experimental unit. The University of Iowa Institutional Review Board has ruled that approval is not needed for use of de-identified and pooled extracted teeth for research purposes.
Beverage exposure
Five teeth, each tooth having one window, were randomly assigned to each beverage. Teeth were suspended with windows submerged in 250 ml beverage at room temperature. Beverages were stirred using a magnetic stir bar. Teeth were rinsed with water every 5 hours and resuspended in fresh beverage for a total of 25 hours exposure. Following exposure, the teeth were removed from the beverage and rinsed.
Measurements
Exposed teeth were mounted in a mandrel with sticky wax leaving the window exposed and protruding from the mandrel.17 Teeth were sectioned (n = 4 sections per tooth) through the window using a microtome (Series 1000 Hard Tissue Microtome, SciFab, Lafayette, CO, 1996). The 100–150 micron wide sections were removed from the tooth and stored in water prior to viewing.
A polarized light microscope (Olympus BX-50, Olympus America Inc, Center Valley, PA, 1996) on 4x magnification was used to visualize the sections.17 Images of four representative sections per tooth surface were photographed (Spot RT Color Video Camera, software v3.1, Diagnostic Instruments, Sterling Heights, MI, 2000).
The Image Pro Plus system (v5.1, Media Cybernetics, Inc, Silver Spring, MD, 2004) was used to measure depth of enamel lesions.17 Lesion depth was defined as the average distance between a straight line representing the original tooth structure and a line drawn at the base of demineralization. Four lesion depths per tooth surface were averaged to create a tooth value.
Statistical Analysis
Statistical analyses were conducted using SAS for Windows (v.9.1 SAS Institute Inc, Cary, NC, USA). Physiochemical properties and lesion depths were reported as means and standard deviations. The two-sample t-test was used to identify differences in physiochemical properties and lesion depths following exposure to UK and US beverages. Pearson correlation and Spearman’s rank correlation tests were used to identify relationships between lesion depths and pH, titratable acidity or fluoride concentrations. The general linear model procedure was used to predict lesion depths from physiochemical properties and country of origin. The level of significance chosen was p< 0.05.
Results
Physiochemical properties were compared between similar beverages from the UK and US. The pH was statistically lower in UK apple juice, orange juice, Diet Pepsi® and Sprite Zero®, and higher in UK orange soda and diet orange soda than in similar US beverages (Table 1). Titratable acidities were statistically higher in UK apple juice, orange juice, orange soda, diet orange soda and Sprite®, and lower in UK Sunny D® than in their US counterparts (Table 2). Fluoride concentrations were statistically lower in UK apple juice, orange juice, Coke®, and Diet Coke®, Sprite® and Sprite Zero®, and higher in UK orange soda, diet orange soda, Pepsi® and Diet Pepsi® than in their US counterparts (Table 3).
Table 1.
pHs* of similar United Kingdom and United States beverages.
| Beverage | United Kingdom |
United States | p-value† |
|---|---|---|---|
| Apple Juice | 3.30 ± 0.05 | 3.43 ± 0.06 | 0.039 |
| Orange Juice | 3.67 ± 0.03 | 3.83 ± 0.03 | 0.002 |
| Coke® | 2.38 ± 0.08 | 2.38 ± 0.08 | 0.999 |
| Diet Coke® | 2.85 ± 0.05 | 2.92 ± 0.03 | 0.116 |
| Orange Soda‡ | 3.03 ± 0.06 | 2.57 ± 0.08 | 0.001 |
| Diet Orange Soda§ | 2.90 ± 0 | 2.73 ± 0.06 | 0.038 |
| Pepsi® | 2.33 ± 0.03 | 2.38 ± 0.03 | 0.101 |
| Diet Pepsi® | 2.58 ± 0.08 | 2.73 ± 0.03 | 0.034 |
| Sprite® | 2.85 ± 0.13 | 2.82 ± 0.06 | 0.710 |
| Sprite Zero® | 2.62 ± 0.03 | 3.03 ± 0.06 | <0.001 |
| Sunny D® | 2.88 ± 0.15 | 2.70 ± 0.13 | 0.191 |
Mean ± SD; n = 3.
Two sample t-test
United Kingdom: Tango®; United States: Fanta®
United Kingdom: Diet Tango®; United States: Fanta Zero®
Table 2.
Titratable acidities* of similar United Kingdom and United States beverages.
| Beverage | United Kingdom ml KOH |
United States ml KOH |
p-value† |
|---|---|---|---|
| Apple Juice | 5.32 ± 0.07 | 2.93 ± 0.02 | <0.001 |
| Orange Juice | 7.16 ± 0.04 | 5.81 ± 0.20 | <0.001 |
| Coke® | 3.05 ± 0.27 | 3.06 ± 0.16 | 0.945 |
| Diet Coke® | 2.90 ± 0.19 | 2.85 ± 0.31 | 0.845 |
| Orange Soda† | 4.70 ± 0.21 | 3.86 ± 0.19 | 0.007 |
| Diet Orange Soda§ | 5.50 ± 0.28 | 4.12 ± 0.20 | 0.002 |
| Pepsi® | 3.03 ± 0.42 | 2.93 ± 0.51 | 0.806 |
| Diet Pepsi® | 3.00 ± 0.20 | 2.80 ± 0.70 | 0.659 |
| Sprite® | 4.07 ± 0.15 | 3.10 ± 0.10 | <0.001 |
| Sprite Zero® | 4.23 ± 0.29 | 3.43 ± 0.72 | 0.150 |
| Sunny D® | 4.20 ± 0 | 6.00 ± 0 | <0.001 |
Mean ± SD of ml 1 M KOH required to bring 50 mL beverage to neutral pH; n = 3.
Two sample t-test
United Kingdom: Tango®; United States: Fanta®
United Kingdom: Diet Tango®; United States: Fanta Zero®
Table 3.
Fluoride concentrations* of similar United Kingdom and United States beverages.
| Beverage | United Kingdom ppm |
United States ppm |
p-value† |
|---|---|---|---|
| Apple Juice | 0.15 ± 0.02 | 0.51 ± 0 | <0.001 |
| Orange Juice | 0.10 ± 0.01 | 0.34 ± 0.02 | <0.001 |
| Coke® | 0.11 ± 0.01 | 0.52 ± 0.01 | <0.001 |
| Diet Coke® | 0.11 ± 0.01 | 0.72 ± 0.01 | <0.001 |
| Orange Soda† | 1.07 ± 0.02 | 0.21 ± 0 | <0.001 |
| Diet Orange Soda§ | 1.15 ± 0.02 | 0.69 ± 0.01 | <0.001 |
| Pepsi® | 0.96 ± 0.01 | 0.09 ± 0.01 | <0.001 |
| Diet Pepsi® | 0.98 ± 0.01 | 0.09 ± 0.02 | <0.001 |
| Sprite® | 0.17 ± 0.00 | 0.69 ± 0.01 | <0.001 |
| Sprite Zero® | 0.16 ± 0.01 | 0.71 ± 0.00 | <0.001 |
| Sunny D® | 0.08 ± 0 | 0.09 ± 0.01 | 0.057 |
Mean ± SD ppm; n = 3.
Two sample t-test
United Kingdom: Tango®; United States: Fanta®
United Kingdom: Diet Tango®; United States: Fanta Zero®
Lesion depths of tooth windows were measured following 25 hours of exposure to similar UK and US beverages (Table 4). Lesion depths were statistically higher in UK apple juice, orange juice, Diet Coke®, Sprite® and Sprite Zero® than in their US counterparts. Lesion depths did not differ between UK and US Coke®, orange soda, diet orange soda, Pepsi®, Diet Pepsi® or Sunny D®.
Table 4.
Lesion depths of teeth exposed to similar United Kingdom and United States beverages for 25 hours.
| Beverage | United Kingdom (µm) |
United States (µm) |
p-value† |
|---|---|---|---|
| Apple Juice | 139 ± 11 | 105 ± 19 | 0.010 |
| Orange Juice | 102 ± 22 | 69 ± 14 | 0.023 |
| Coke® | 148 ± 28 | 179 ± 22 | 0.090 |
| Diet Coke® | 152 ± 21 | 91 ± 12 | <0.001 |
| Orange Soda† | 112 ± 20 | 127 ± 23 | 0.278 |
| Diet Orange Soda§ | 216 ± 42 | 184 ± 38 | 0.250 |
| Pepsi® | 147 ± 39 | 140 ± 30 | 0.763 |
| Diet Pepsi® | 211 ± 89 | 137 ± 33 | 0.115 |
| Sprite® | 143 ± 38 | 88 ± 30 | 0.033 |
| Sprite Zero® | 225 ± 29 | 132 ± 20 | <0.001 |
| Sunny D® | 189 ± 28 | 170 ± 16 | 0.216 |
Mean ± SD µm; n = 5 teeth. Lesion depths of 4 sections/tooth were averaged to get a single lesion depth for each tooth
Two sample t-test
United Kingdom: Tango®; United States: Fanta®
United Kingdom: Diet Tango®; United States: Fanta Zero®
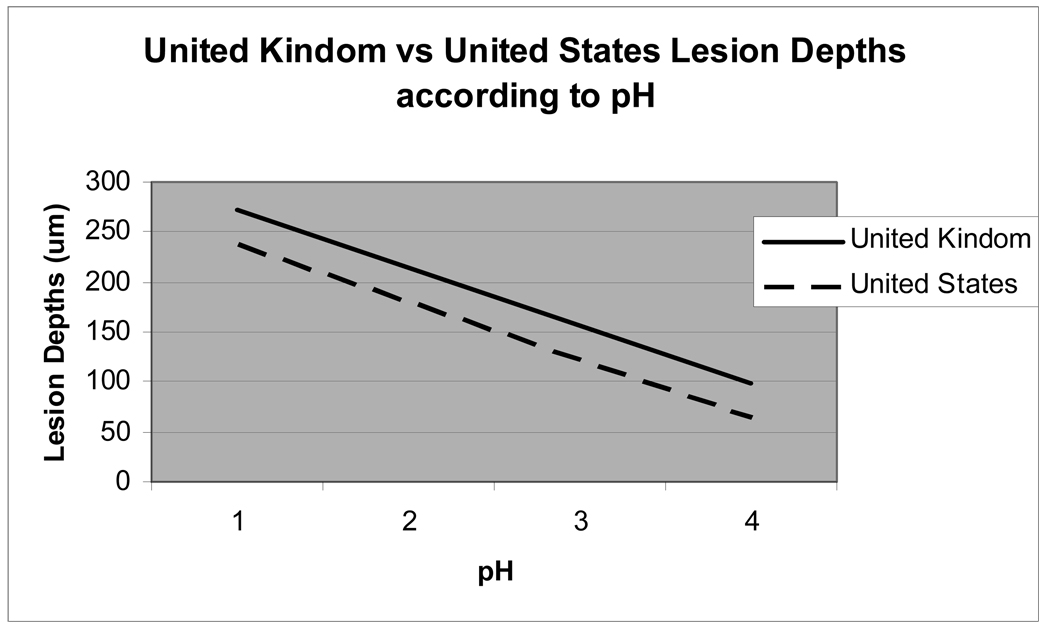
Associations between lesion depths and pH, titratable acidity and fluoride concentrations were explored using generalized linear regression models. Lesion depths were associated with pH (p=0.010) and country of origin (p=0.002) as shown in Figure 1. Lesion depths were not associated with either the beverage fluoride concentration or titratable acidity.
Figure 1.
Lesion depths by pH for country of origin.
Discussion
The results reported herein suggest that under similar laboratory conditions some, but not all, beverages available in the UK have higher erosion potentials than those available in the US. Neither titratable acidities nor fluoride concentrations were associated with the erosion potentials; however, initial pHs were associated with erosion potentials. Results reported herein are unique in that the UK and US beverages were compared under identical conditions within the same time frame.
The erosion potential of beverages has been associated with pH, titratable acidity and calcium and fluoride concentrations. Beverage pH is considered a stronger predictor of erosion potential than titratable acidity. Larsen and Nyvad reported that lesion depth was associated with initial beverage pH, but not buffering ability, following 24 hours of enamel exposure to the beverage.13 Barbour et al reported a linear relationship between pH and enamel hardness following a 120 second exposure to citric acid solutions with pHs ranging between 2.3 and 6.0.24 Jensdottir et al reported that calcium released during a 24 hour beverage exposure was associated with initial pH, while enamel weight loss was associated with both pH and titratable acidity.14 The same group subsequently reported that the immediate erosion potential was associated with pH and not titratable acidity.21 Hjortsjö et al reported that stannous fluoride and hydrofluoric acid solutions, but not sodium fluoride or titanium tetrafluoride solutions, protected against in vivo erosion associated with citric acid exposure.25 Rios et al reported that concentrated dentifrice did not protect bovine enamel from a cola erosive challenge in situ.26
Lesion depths were greater in both natural (i.e., apple and orange juices) and processed (i.e., Diet Coke, Sprite and Sprite Zero) beverages from the UK compared to the US. The pH was lower and titratable acidity higher for both UK apple and orange juices measured in this laboratory. Differences in pH and titratable acidity between brands of juices measured within the same laboratory has been reported by Willerhousen for apple juices from Germany27 and by Jensdottir for orange juices from Denmark,21 and suggest that different varieties of fruits used to produce juices influence these characteristics. The pHs reported herein for UK apple and orange juice are consistent with those previously reported for apple (3.28–3.83; N=11) and orange (3.12–4.08; n=10) juice.21,27 The titratable acidity reported herein for orange juice was higher than reported for orange juices (1600–4450 µl);21 comparable titratable acidities of apple juices are not available. Data on pHs and titratable acidities of multiple brands of US apple and orange juices are not currently available; thus, we do not know if the brands analyzed in this study are representative of US beverages. The processed beverages selected for this study are produced locally from company formulas, and it is unclear as to why differences in pH and titratable acidity exist.
Beverage fluoride concentrations reflect the fluoride concentration of water used in their production. Either natural or artificial fluoridation of water systems could have contributed to concentrations observed in the beverages reported on herein. The concentrations of fluoride in beverages studied herein were not associated with lesion depths, suggesting that the fluoride concentrations were either below the protective threshold or did not encompass a range sufficient to detect a protective effect. The results reported herein are consistent with those of Hara and Zero who reported it was not possible to detect protective effects of fluoride given the narrow range of fluoride concentrations within their beverages.28
Consistent with other researchers, pH was a primary predictor of erosion potential in the current study. More surprising, though, was the finding that country of origin was predictive of erosion potential in modeling analyses. UK beverages with higher erosion potentials did not necessarily have lower pHs than their US counterparts. This finding is not readily explained by the present analyses, and it is hypothesized that the differences in manufacturers’ formulations leading to differences in erosion potentials between beverages available in the UK and US could contribute to differences in clinically apparent erosion thought to exist between European countries and the US.
Beyond differences inherent to beverages, different dietary habits and food patterns are also thought to contribute to clinical erosion differences. West et al reported that tooth loss increased with increasing temperature and duration of exposure.29 Anecdotal reports suggest that Europeans tend to consume beverages at room temperature, while beverages are typically consumed cold in the US. Within the US, acidic beverages are often consumed throughout the day and held within the mouth, increasing exposure time. Furthermore, acidic beverages are one component of the erosive process; additive effects of acid foodstuffs (e.g., pickled foods, sour candies, fruits) likely contribute to erosion, while protective effects of neutral or calcium-containing foods (e.g., milk, calcium-fortified foods, nuts) could limit risk of erosion. Although speculative, dietary habits and food patterns deserve consideration as contributors to different erosion patterns observed between populations.
Calcium concentrations of beverages used in this study were not assayed; however, all products are naturally low in calcium, and none of the products were fortified with calcium. Thus, it is unlikely that calcium was present in a sufficient concentration to influence the erosion process. The study was in vitro, and did not allow for consideration of individual differences in swallowing, salivary clearance or remineralization between exposures. UK beverages were shipped unopened in their original containers. Upon arrival, they were stored according to manufacturer’s guidelines. US beverages were purchased within a similar time frame; however, they were not exposed to shipping conditions.
Conclusions
Differences were noted for pH, titratable acidity and erosion potentials between beverages available in the UK and the US. Although pH was negatively associated with erosion potential, pH did not explain the differences between countries. Additional investigation is necessary to identify if product formulations contribute to different clinical erosion patterns observed between Europe and the US.
Acknowledgements
The authors thank Maggie Hogan, Jeffrey Harless and Judy Heilman for their technical assistance in the laboratory. Portions of this study were presented and published in abstract form at the General Sessions of the International Association for Dental Research in Dallas TX (2008).
This study was supported by NIDCR Grant T32 DEO14678-05. The contents are the responsibility of the authors and do not necessarily reflect the views of the granting organizations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sarah Murrell, College of Dentistry, University of Iowa, Dental Science Building, Iowa City, 52242-1010..
Teresa A. Marshall, Department of Preventive and Community Dentistry, College of Dentistry, University of Iowa, N-335 Dental Science Building, Iowa City, Iowa 52242-1010..
Paula J. Moynihan, Institute for Aging and Health, School of Dental Sciences; Newcastle University upon Tyne NE2 4BW, UK..
Fang Qian, Department of Dows Research, N-431 Dental Science Building, College of Dentistry, Iowa City, Iowa 52242-1010..
James S. Wefel, Dows Institute for Dental Research, N-413 Dental Science Building, College of Dentistry, Iowa City, Iowa 52242-1010..
References
- 1.Lussi A, Jaeggi T, Zero D. The role of diet in the aetiology of dental erosion. Caries Research. 2004;38:34–44. doi: 10.1159/000074360. [DOI] [PubMed] [Google Scholar]
- 2.Smith BG, Bartlett DW, Robb ND. The prevalence, etiology and management of tooth wear in the United Kingdom. Journal of Prosthodontic Dentistry. 1997;78:367–372. doi: 10.1016/s0022-3913(97)70043-x. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett D, Phillips K, Smith B. A difference in perspective – the North American and European interpretations of tooth wear. International Journal of Prosthodontics. 1999;12:401–408. [PubMed] [Google Scholar]
- 4.Gandara BK, Truelove EL. Diagnosis and management of dental erosion. Journal of Contemporary Dental Practice. 1999;1:1–17. [PubMed] [Google Scholar]
- 5.Dugmore CR, Rock WP. The prevalence of tooth erosion in 12-year-old children. British Dental Journal. 2004;196:279–282. doi: 10.1038/sj.bdj.4811040. [DOI] [PubMed] [Google Scholar]
- 6.El Aidi H, Bronkhorst EM, Truin GJ. A longitudinal study of tooth erosion in adolescents. Journal of Dental Research. 2008;87:731–735. doi: 10.1177/154405910808700813. [DOI] [PubMed] [Google Scholar]
- 7.Harding MA, Whelton H, O’Mullane DM, Cronin M. Dental erosion in 5-year-old Irish school children and associated factors: a pilot study. Community Dental Health. 2003;20:165–170. [PubMed] [Google Scholar]
- 8.Chadwick BL, White DA, Morris AJ, Evans D, Pitts NB. Non-carious tooth conditions in children in the UK, 2003. British Dental Journal. 2006;200:379–384. doi: 10.1038/sj.bdj.4813424. [DOI] [PubMed] [Google Scholar]
- 9.Milosevic A, Bardsley PF, Taylor S. Epidemiological studies of tooth wear and dental erosion in 14-year old children in North West England. Part 2: the association of diet and habits. British Dental Journal. 2004;197:479–483. doi: 10.1038/sj.bdj.4811747. [DOI] [PubMed] [Google Scholar]
- 10.Deery C, Wagner ML, Longbottom C, Simon R, Nugent ZJ. The prevalence of dental erosion in a United States and a United Kingdom sample of adolescents. Pediatric Dentistry. 2000;22:505–510. [PubMed] [Google Scholar]
- 11.Mathew T, Casamassimo PS, Hayes JR. Relationship between sports drinks and dental erosion in 304 university athletes in Columbus, Ohio, USA. Caries Research. 2002;36:281–287. doi: 10.1159/000063927. [DOI] [PubMed] [Google Scholar]
- 12.McGuire J, Szabo A, Jackson S, Bradley TG, Okunseri C. Erosive tooth wear among children in the United States: relationship to race/ethnicity and obesity. International Journal of Paediatric Dentistry. 2009;19:91–98. doi: 10.1111/j.1365-263X.2008.00952.x. [DOI] [PubMed] [Google Scholar]
- 13.Larsen MJ, Nyvad B. Enamel erosion by some soft drinks and orange juices relative to their pH, buffering effect and contents of calcium phosphate. Caries Research. 1999;33:81–87. doi: 10.1159/000016499. [DOI] [PubMed] [Google Scholar]
- 14.Jensdottir T, Bardow A, Holbrook P. Properties and modification of soft drinks in relation to their erosive potential in vitro. Journal of Dentistry. 2005;33:569–575. doi: 10.1016/j.jdent.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Hughes JA, West NX, Parker DM, Newcombe RG, Addy M. Development and evaluation of a low erosive blackcurrant juice drink. 3. Final drink and concentrate, formulae comparisons in situ and overview of the concept. Journal of Dentistry. 1999;27:345–350. doi: 10.1016/s0300-5712(98)00068-2. [DOI] [PubMed] [Google Scholar]
- 16.West NX, Hughes JA, Parker DM, Moohan M, Addy M. Development of low erosive carbonated fruit drinks 2. Evaluation of an experimental carbonated blackcurrant drink compared to a conventional carbonated drink. Journal of Dentistry. 2003;31:361–365. doi: 10.1016/s0300-5712(03)00046-0. [DOI] [PubMed] [Google Scholar]
- 17.Davis RE, Marshall TA, Qian F, Warren JJ, Wefel JS. In vitro protection against dental erosion afforded by commercially available, calcium-fortified 100 percent juices. Journal of the American Dental Association. 2007;138:1593–1598. doi: 10.14219/jada.archive.2007.0109. [DOI] [PubMed] [Google Scholar]
- 18.Johansson AK, Lingström P, Birkhed D. Comparison of factors potentially related to the occurrence of dental erosion in high-and low-erosion groups. European Journal of Oral Sciences. 2002;110:204–211. doi: 10.1034/j.1600-0447.2002.11211.x. [DOI] [PubMed] [Google Scholar]
- 19.Hemingway CA, Parker DM, Addy M, Barbour ME. Erosion of enamel by non-carbonated soft drinks with and without toothbrushing abrasion. British Dental Journal. 2006;207:447–450. doi: 10.1038/sj.bdj.4814073. [DOI] [PubMed] [Google Scholar]
- 20.Parry J, Shaw L, Arnaud MJ, Smith AJ. Investigation of mineral waters and soft drinks in relation to dental erosion. Journal of Oral Rehabilitation. 2001;28:766–772. doi: 10.1046/j.1365-2842.2001.00795.x. [DOI] [PubMed] [Google Scholar]
- 21.Jensdottir T, Holbrook P, Nauntofte B, Buchwald C, Bardow A. Immediate erosive potential of cola drinks and orange juices. Journal of Dental Research. 2006;85:226–230. doi: 10.1177/154405910608500304. [DOI] [PubMed] [Google Scholar]
- 22.Ehlen LA, Marshall TA, Qian F, Wefel JS, Warren JJ. Acidic beverages increase the risk of in vitro tooth erosion. Nutrition Research. 2008;28:299–303. doi: 10.1016/j.nutres.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owens BM. The potential effects of pH and buffering capacity on dental erosion. General Dentistry. 2007;55:527–531. [PubMed] [Google Scholar]
- 24.Barbour ME, Parker DM, Allen GC, Jandt KD. Human enamel dissolution in citric acid as a function of pH in the range of 2.30 ≤ pH ≤ 6.30 – a nanoindentation study. European Journal of Oral Sciences. 2003;111:258–262. doi: 10.1034/j.1600-0722.2003.00039.x. [DOI] [PubMed] [Google Scholar]
- 25.Hjorstjö C, Jonski G, Thrane PS, Saxegaard E, Young A. The effects of acidic fluoride solutions on early enamel erosion in vivo. Caries Research. 2009;43:126–131. doi: 10.1159/000209345. [DOI] [PubMed] [Google Scholar]
- 26.Rios D, Magalhães AC, Polo ROB, Wiegand A, Attin T, Buzalaf MAR. The efficacy of a highly concentrated fluoride dentifrice on bovine enamel subjected to erosion and abrasion. Journal of the American Dental Association. 2008;139:1652–1656. doi: 10.14219/jada.archive.2008.0107. [DOI] [PubMed] [Google Scholar]
- 27.Willershausen B, Callaway A, Azrak B, Duschner H. Influence of apple juice on human enamel surfaces of the first and second dentition – an in vitro study. European Journal of Medical Research. 2008;13:349–354. [PubMed] [Google Scholar]
- 28.Hara AT, Zero DT. Analysis of the erosive potential of calcium-containing acidic beverages. European Journal of Oral Sciences. 2008;116:60–65. doi: 10.1111/j.1600-0722.2007.00513.x. [DOI] [PubMed] [Google Scholar]
- 29.West NX, Hughes JA, Addy M. Erosion of dentine and enamel in vitro by dietary acids: the effect of temperature, acid character, concentration and exposure time. Journal of Oral Rehabilitation. 2000;27:875–880. doi: 10.1046/j.1365-2842.2000.00583.x. [DOI] [PubMed] [Google Scholar]