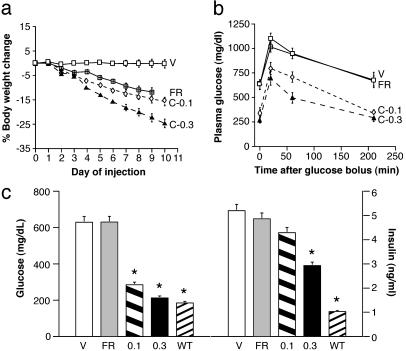
Fig. 2.
Treatment with CNTFAx15 decreases body weight and improves glucose tolerance. Male db/db mice treated with either vehicle (V), CNTFAx15 at 0.1 or 0.3 mg/kg per day (C-0.1 or C-0.3), or pair-fed to the C-0.3 treatment group (FR). (a) Daily body weight changes are shown as the percent difference from the first day of injection. (b) An oral glucose tolerance test was conducted 24 hr after the last treatment, and serum glucose was measured at the indicated times after oral gavage. All data are expressed as mean (n ≥ 6) ± SEM. (c) Fasting serum glucose (mg/dl) and insulin (ng/ml) levels (mean ± SEM, n = 6-10) for each group and from lean, nondiabetic littermates (WT). Tolerance test, C-0.1 vs. vehicle (F1,3 = 114.8, P < 0.001) and C-0.3 vs. vehicle (F1,3 = 78.8, P < 0.001). For glucose P < 0.001 and for insulin P < 0.05. * indicates the difference between ad lib-fed db/db vehicle and FR controls by Dunnett's post hoc test.