Fig. 6.
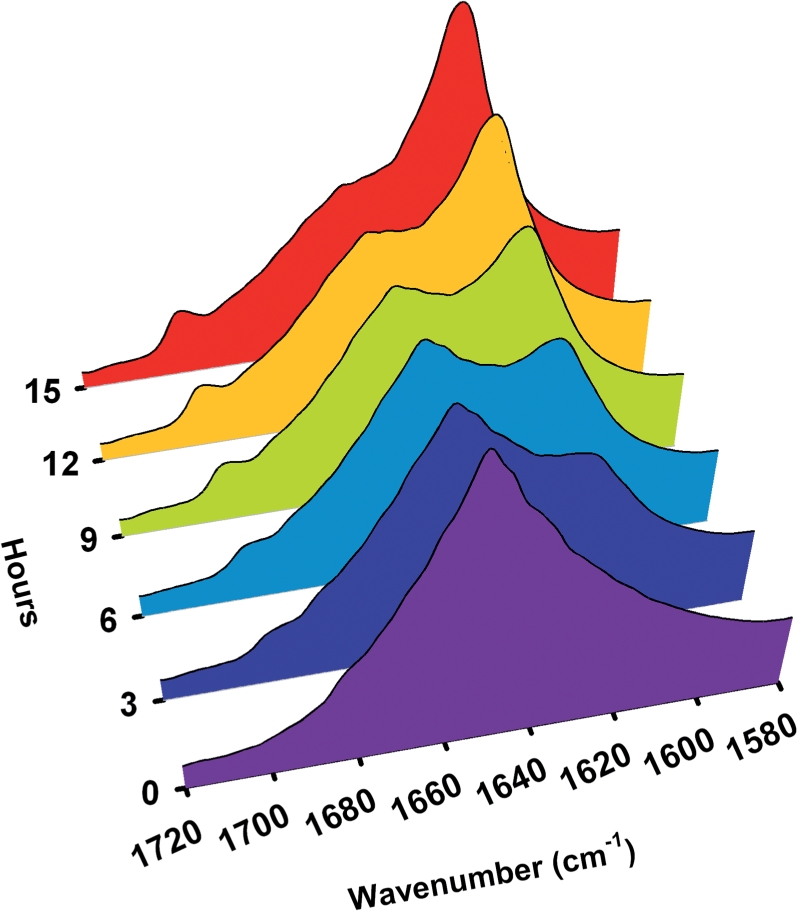
Real-time FTIR spectra of poly-l-lysine to analyze the effect of slow drying (65% RH) on the protein secondary structures. The fully hydrated poly-l-lysine changes their conformation from random coil to extended β-sheet from 0 to 15 h (0 h, purple; 3 h, blue, 6 h, cyan; 9 h, light green; 12 h, orange, 15 h, red). Arrowheads indicate 1,625, 1,651 and 1,693 cm−1 for signatures of random coil and extended β-sheet.
