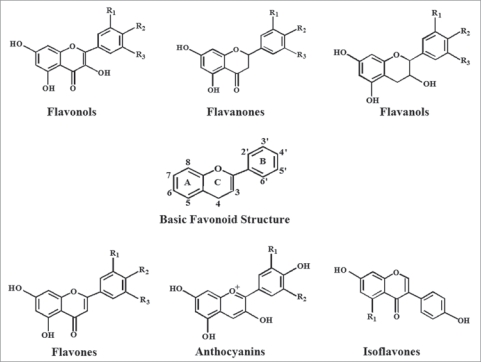
Figure 3.
Chemical structures of sub-classes of flavonoids. Based on the variation in the type of heterocycle involved, flavonoids are divided into six major subclasses: flavonols, flavanones, flavanols, flavones, anthocyanins and isoflavones. Individual differences within each group arise from the variation in number and arrangement of the hydroxyl groups and their extent of alkylation and/or glycosylation. Flavonols (such as quercetin and kaempferol), have a 3-hydroxy pyran-4-one group on the C ring. Flavanones (such as naringenin and taxifolin), have an unsaturated carbon-carbon bond in the C ring. Flavanols (such as the catechins), lack both a 3-hydroxyl group and the 4-one structure in the C ring. Flavones (such as luteolin), lack a hydroxyl group in the 3-position on the C ring. Anthocyanins (such as cyanidin), are characterized by the presence of an oxonium ion on the C ring and are highly coloured as a consequence and in isoflavones (such as genistein), the B ring is attached to the C ring in the 3-position, rather than the 2-position as is the case with the other flavonoids.