Figure 1.
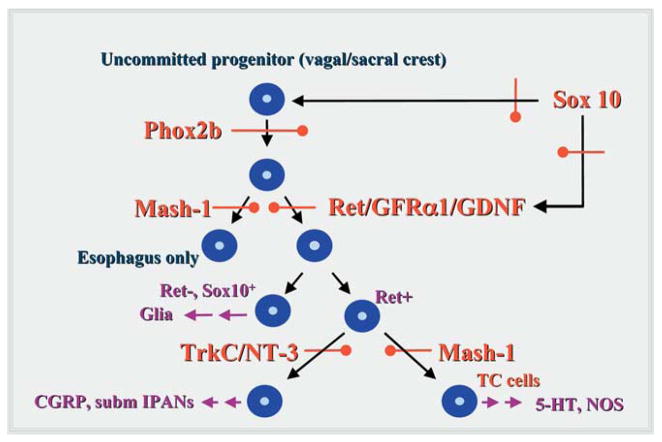
Specific transcription and growth factors define stages in ENS development. The earliest precursors of enteric neurons and glia are multipotent and dependent on the expression of the transcription factor, Phox2b. Derivatives of these cells that colonize all regions of the bowel, with the exception of the primordial esophagus and adjacent stomach, depend on the stimulation of Ret by GDNF in a complex with the Ret coreceptor, GFRα1. The ENS of the primordial esophagus and adjacent stomach is Ret-independent, and depends instead on the expression of the b-HLH transcription factor, Mash-1. Both the expression of Phox2b and Ret are promoted by the transcription factor, Sox10, the expression of which is essential for ENS development. Because they are required by multipotent precursors early in development, loss-of-function mutations in Phox2b, Sox10, or Ret (or its ligand or coreceptor) give rise to aganglionosis of the bowel. Only the esophagus and adjacent stomach become aganglionic due to loss-of-function mutations in Mash-1. Later stages in ENS ontogeny involve the development of committed lineages of enteric neuronal or glial precursors. Sets of enteric neurons that colonize the bowel distal to the esophagus and adjacent stomach are Mash-1-dependent or -independent. Loss-of function mutations in Mash-1 thus do not cause the gut to become aganglionic, but to lose only specific derivatives of Mash-1-dependent precursors, including transiently catecholaminergic (TC) precursors and the 5-HT- and NOS-containing neurons to which they give rise. A subset of Mash-1-independent precursors expresses TrkC (evidently in response to BMP-2 and/or -4 stimulation) and thus becomes NT-3-dependent. These cells include the submucosal IPANs that initiate peristaltic and secretory reflexes.