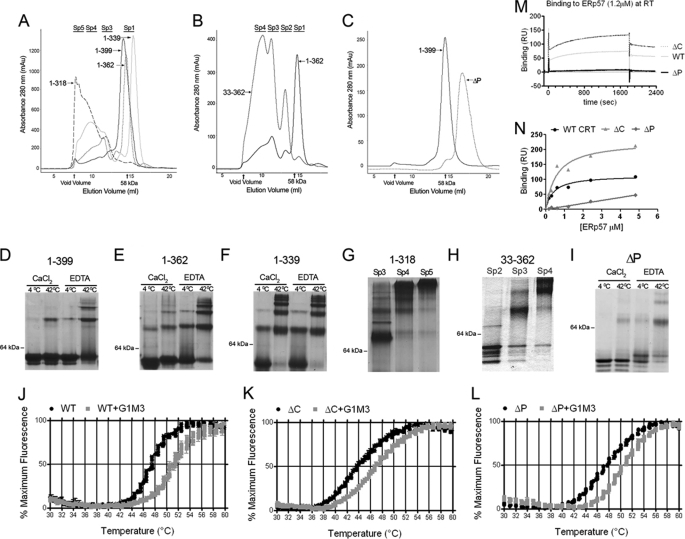
FIGURE 1.
Impacts of calreticulin domains on structure and functional activities. Analytical gel-filtration chromatography of C-terminal (A), N-terminal (B), and P-domain (C) truncation mutants of mCRT. All constructs were directly analyzed following nickel affinity chromatography, except mCRT(ΔP) for which monomers were first isolated by gel filtration and then re-analyzed by a second analytical gel-filtration step shown. Following gel-filtration chromatography, peak fractions were analyzed by native-PAGE following protein (12 μm) incubations for 1 h in 1 mm CaCl2 or 10 mm EDTA at 4 or 42 °C (D–F and I). Fractions corresponding to the indicated oligomeric species (Sp2–Sp5) of mCRT1–318 or mCRT33–362 were directly analyzed by native PAGE (1 mm CaCl2, 4 °C) (G and H). One representative analysis is shown of two or more independent analyses. J–L, monomeric mCRT constructs were incubated with the fluorophore SYPRO Orange and subjected to a thermal stability analysis using a real-time PCR machine. Normalized fluorescence data (mean of triplicate wells ± S.E.) for one of three independent experiments are shown. Compiled Tm values for all constructs discussed in this report are shown in supplemental Table S1. M and N, mCRT constructs were immobilized on a Biacore CM5 chip to response unit (RU) values of 438, 426, and 480 (respectively, for WT, ΔP, and ΔC), and human ERp57 was injected over each surface or a control bovine serum albumin surface. Representative sensorgrams following subtraction of corresponding sensorgrams from the bovine serum albumin surface are shown in M. Dose-response graphs for binding constant calculations are shown in N. One representative of two (ΔC and ΔP) or five (WT) independent analyses of N are shown.