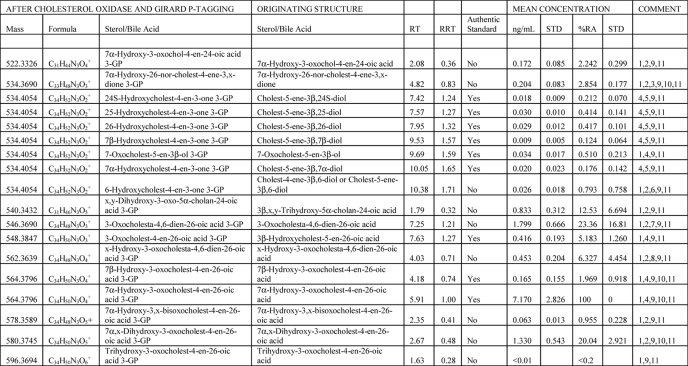
TABLE 1.
Oxysterols and bile acids in CSF
The following abbreviations were used: RT, retention time/min; RRT, retention time relative to 7α-hydroxy-3-oxocholest-4-en-26-oic acid; STD, S.D.; %RA, % relative abundance (7α-hydroxy-3-oxocholest-4-en-26-oic acid = 100%, n = 9).
1 Quantitative estimate was based on 24(RS)-[26,26,26,27,27,27-2H6]hydroxycholesterol internal standard. Mean concentration was ± S.D. (n = 6).
2 Identification was based on exact mass and MSn spectra.
3 26-Norsterol is a likely decomposition product of a 24-oxo-26-acid. Alternatives to the oxo group x are an enol or epoxy group; all add 14 Da to the sterol structure.
4 Identification was based on comparison with authentic standards.
5 Quantification was based on 24(RS)-[26,26,26,27,27,27-2H6]hydroxycholesterol internal standard. Mean concentration was ± S.D. (n = 6).
6 Cholest-4-ene-3β,6-diol and/or cholest-5-ene-3β,6-diol are decomposition products of 5,6-epoxycholestan-3β-ol and cholestane-3β,5α,6β-triol.
7 3-Oxocholesta-4,6-dien-26-oic acid is a dehydration product of 7α-hydroxy-3-oxocholest-4-en-26-oic acid.
8 x-Hydroxy-3-oxocholesta-4,6-dien-26-oic acid is a likely dehydration product of 7α-x-dihydroxy-3-oxocholest-4-en-26-oic acid.
9 Retention time/min (RT) for compounds eluting in chromatograms shown in Figs. 1–4. Relative retention time (RRT, mean) was to 7α-hydroxy-3-oxocholest-4-en-26-oic acid.
10 Resolved syn and anti conformers.
11 %RA against 7α-hydroxy-3-oxocholest-4-en-26-oic acid (n = 9).