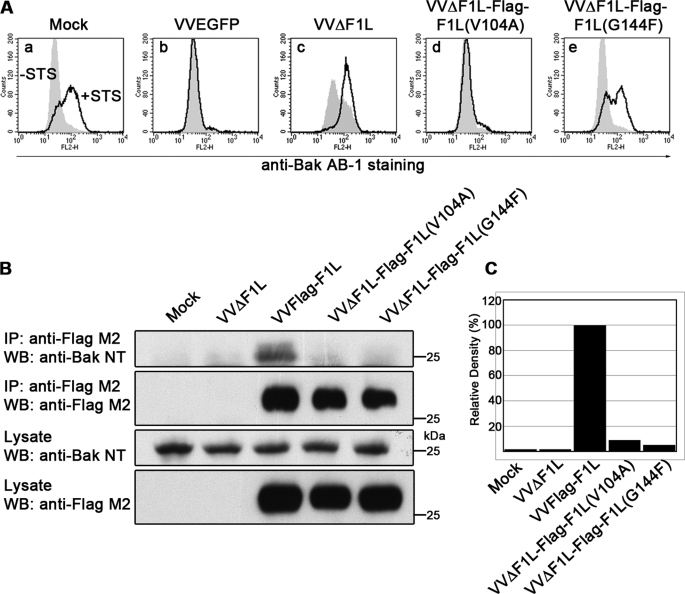
FIGURE 3.
The BH1 domain of F1L is critical for its antiapoptotic ability. A, Jurkat cells were mock-infected or infected with VVEGFP, VVΔF1L, VVΔF1L-FLAG-F1L(V104A), or VVΔF1L-FLAG-F1L(G144F) at an m.o.i. of 10 for 4 h. Cells were then treated with 250 nm STS for 1.5 h to induce apoptosis. Bak N-terminal exposure was monitored by staining cells with the conformation-specific anti-Bak AB-1 antibody. Shaded histograms, untreated cells; open histograms, STS-treated cells. B, HEK 293T cells were mock-infected or infected with VVΔF1L, VVFLAG-F1L, VVΔF1L-FLAG-F1L(V104A), or VVΔF1L-FLAG-F1L(G144F) at an m.o.i. of 5 for 12 h. FLAG-tagged constructs were immunoprecipitated with anti-FLAG M2, and both immunoprecipitates (IP) and lysates were Western-blotted (WB) with anti-FLAG M2 (20% of samples) and anti-Bak NT (30% of samples). C, quantification of the amount of endogenous Bak co-immunoprecipitated with each FLAG-tagged construct in Fig. 3B, top panel, is shown.