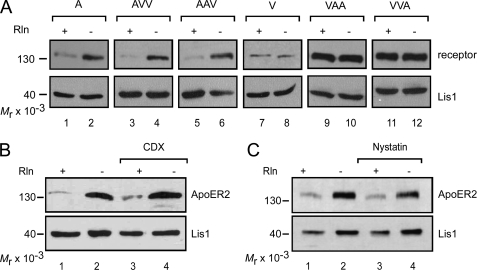
FIGURE 7.
The extracellular domain of ApoER2 but not its sorting to lipid rafts is required for Reelin-induced lysosomal receptor degradation. A, 3T3 cells expressing one of the wild type (A and V) or chimeric receptors (AVV, AAV, VAA, and VVA) were stimulated with RCM (lanes 1, 3, 5, 7, 9, and 11) or MCM (lanes 2, 4, 6, 8, 10, and 12) for 5 h and analyzed for receptor degradation by Western blotting using Ab 20 for ApoER2, VAA, and VVA; Ab 220 for AAV and AVV; and Ab 74 for VLDLR. Lis1 was highlighted using an anti-Lis1 antibody and used as a loading control. B, ApoER2-expressing 3T3 fibroblasts were incubated with RCM (lanes 1 and 3) or MCM (lanes 2 and 4) and the raft-disrupting agent CDX (5 mm; lanes 3 and 4) for 5 h. Cell extracts were analyzed for ApoER2 degradation by Western blotting using Ab 20 in combination with an HRP-coupled goat-anti-rabbit antibody. Lis1 was highlighted using an anti-Lis1 antibody and used as a loading control. C, cells were treated and analyzed as described for B, except that 15 μg/ml nystatin was used for disruption of rafts.