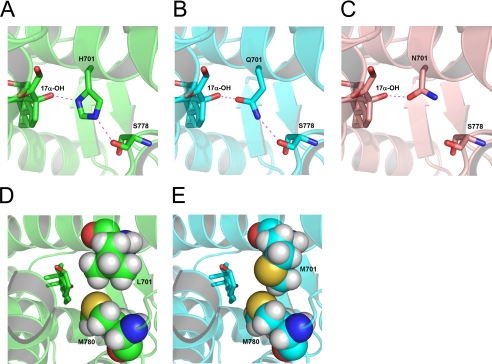
FIGURE 5.
Conserved hydrogen-bonding network around position 701 defines the cortisol response. A, the crystal structure of the AR L701H mutant (33) shows an H-bonding network between the 17α-OH group of 9α-fluorocortisol and the Nδ1 nitrogen of His701 and between the Nϵ2 nitrogen of His701 and the backbone carbonyl of Ser778. B, the same H-bonding network is maintained in the AR L701Q structure with the 17α-OH group interacting with the Oϵ1 oxygen of Gln701 and the Nϵ2 nitrogen of Gln701 forming an H-bond to the backbone of Ser778. C, contrary to this, in the AR L701N structure, an interaction between the mutated residue and the steroid's 17α-OH group is possible, but the interaction with Ser778 is missing. D and E, modeling shows that Met701 moderately improved packing with Met780, including an electrostatically favorable sulfur-sulfur contact (E), compared with the interaction between wild-type Leu701 and Met780 (D).