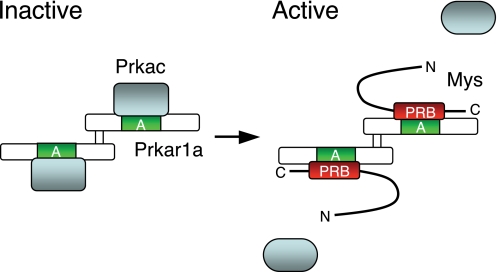
FIGURE 9.
Proposed model of the regulation of PKA activation by Mys. PKA forms an inactive holoenzyme containing a Prkar1a dimer and two Prkacs (left). Prkac binds to domain A of Prkar1a. Mys competes with Prkac for binding to domain A of Prkar1a via its C-terminal PRB domain, resulting in dissociation of Prkacs from a Prkar1a dimer and activation of PKA (right). The active Prkacs phosphorylate protein substrates. A, cAMP-binding domain A; PRB, Prkar1-binding domain.