Abstract
How do cartilaginous elements attain their characteristic size and shape? Two intimately coupled processes underlie the patterned growth of cartilage. The first is histogenesis, which entails the production of cartilage as a discrete tissue; the second is morphogenesis, which pertains to the origins of three-dimensional form. Histogenesis relies on cues that promote the chondrogenic differentiation of mesenchymal cells, whereas morphogenesis requires information that imbues cartilage with stage-specific (e.g. embryonic versus adult), region-specific (e.g. cranial versus appendicular) and species-specific size and shape. Previous experiments indicate that early programmatic events and subsequent signaling interactions enable chondrogenic mesenchyme to undergo histogenesis and morphogenesis, but precise molecular and cellular mechanisms that generate cartilage size and shape remain unclear. In the face and jaws, neural crest-derived mesenchyme clearly plays an important role, given that this embryonic population serves as the source of chondrocytes and of species-specific patterning information. To elucidate mechanisms through which neural crest-derived mesenchyme affects cartilage size and shape, we made chimeras using quail and duck embryos, which differ markedly in their craniofacial anatomy and rates of maturation. Transplanting neural crest cells from quail to duck demonstrates that mesenchyme imparts both stage-specific and species-specific size and shape to cartilage by controlling the timing of preceding and requisite molecular and histogenic events. In particular, we find that mesenchyme regulates FGF signaling and the expression of downstream effectors such as sox9 and col2a1. The capacity of neural crest-derived mesenchyme to orchestrate spatiotemporal programs for chondrogenesis autonomously, and to implement cartilage size and shape across embryonic stages and between species simultaneously, provides a novel mechanism linking ontogeny and phylogeny.
Keywords: Neural crest mesenchyme, Mandibular chondrogenesis, Meckel’s cartilage, Sox9, Col2a1, FGF signaling, Quail-duck chimeras, Evolutionary developmental biology
INTRODUCTION
We hope that our attempts to construct a quantitative theory will stimulate others to delve more deeply below the level of pure phenomenology and come to grips with the central issue underlying evolutionary diversification of size and shape – that is, the morphogenetic unfolding of genetic programs in ontogeny and their alteration in the course of phyletic evolution (Alberch et al., 1979, p. 297).
The generation of size and shape has long been a central topic of developmental and evolutionary biology. In their earliest incarnations, size and shape studies focused primarily on proportional scaling or ‘allometry’ of anatomical structures observed during growth or across species (Thompson, 1917; Huxley, 1932). Such research ultimately begot the field of morphometrics, which has typically used multivariate methods and computer-based algorithms to quantify and visualize differences in size and shape (Bookstein, 1978; Benson et al., 1982; Siegel and Benson, 1982; Bookstein, 1990). Although the ability to measure size and shape has become entirely refined over time, results are often phenomenological, and so many morphometricians have contextualized their data with quantitative genetics or evolutionary developmental theories such as heterochrony, as a means to explain changes in size and shape during ontogeny and phylogeny (Gould, 1966; Alberch et al., 1979; Lande, 1979; Atchley, 1981; McKinney, 1988; Atchley and Hall, 1991). These approaches have been informative, but much remains to be understood regarding specific morphogenetic mechanisms that regulate size and shape. Essential information has begun to emerge from the application of relatively recent techniques in developmental biology, and especially through manipulations that test directly the extent to which molecular and cellular events underlie the spatiotemporal patterning of individual anatomical elements.
For many reasons, including its incomparable paleontological history, its fairly simple geometry, its evolutionary variability and its high degree of visibility during embryogenesis, the vertebrate skeleton has featured prominently in the study of size and shape. In particular, pattern formation in the vertebrate skull has long been the subject of intense investigation (de Beer, 1937; Hanken and Hall, 1993), mainly in relation to genetic specification of skeletal element identity (Balling et al., 1989; Lufkin et al., 1992; Gendron-Maguire et al., 1993; Rijli et al., 1993; Qiu et al., 1997; Schilling, 1997; Hunt et al., 1998; Smith and Schneider, 1998; Grammatopoulos et al., 2000; Pasqualetti et al., 2000; Creuzet et al., 2002; Depew et al., 2002; Kimmel et al., 2005), tissue interactions that mediate mesenchymal differentiation into cartilage and bone (Schowing, 1968; Tyler, 1978; Bee and Thorogood, 1980; Hall, 1980; Hall, 1982; Tyler, 1983; Thorogood et al., 1986; Hall, 1987; Thorogood, 1987; Richman and Tickle, 1989; Richman and Tickle, 1992; Dunlop and Hall, 1995; Ferguson et al., 2000; Shigetani et al., 2000; Couly et al., 2002; Francis-West et al., 2003), regulation of skeletal growth and polarity by secreted molecules (Barlow and Francis-West, 1997; Francis-West et al., 1998; Schneider et al., 2001; Hu et al., 2003; Abzhanov et al., 2004; Abzhanov and Tabin, 2004; Crump et al., 2004; Wilson and Tucker, 2004; Wu et al., 2004; Liu et al., 2005; Marcucio et al., 2005; Wu et al., 2006), and control of species-specific skeletal morphology by mesenchyme (Andres, 1949; Wagner, 1959; Noden, 1983; Schneider and Helms, 2003; Tucker and Lumsden, 2004; Mitsiadis et al., 2006). The role of mesenchyme in conveying species-specific pattern has been recognized principally through inter-specific grafting experiments (Noden and Schneider, 2006; Lwigale and Schneider, 2008). For example, neural crest-derived mesenchyme destined to form bones and cartilages in the face and jaws, was transplanted between quail and duck (Schneider and Helms, 2003; Tucker and Lumsden, 2004). Chimeric ‘quck’ embryos, which are duck hosts with quail donor cells, formed quail-like beaks and jaw joints, whereas chimeric ‘duail’ displayed duck-derived morphology in quail hosts. The precise molecular mechanisms through which mesenchyme accomplishes this complex task remain opaque, but the ability of mesenchyme to regulate its own gene expression and differentiation, as well as that of adjacent tissues such as epithelia, is apparent (Schneider and Helms, 2003; Eames and Schneider, 2005; Schneider, 2005; Merrill et al., 2008).
To identify developmental mechanisms that generate skeletal size and shape, we employed the quail-duck chimeric transplantation system, which exploits the divergent maturation rates and distinct species-specific anatomies of these birds (Fig. 1). We examined the closely associated processes underlying cartilage formation, histogenesis and morphogenesis. Histogenesis dictates tissue characteristics (e.g. biochemical qualities) and involves the differentiation of mesenchyme during two easily observable stages. First, pre-chondrogenic cells distinguish themselves by undergoing condensation, and second, they enter overt chondrification, when they secrete abundant extracellular matrix (Eames et al., 2003; Hall, 2005). Whereas histogenesis concerns cartilage differentiation, morphogenesis encompasses the establishment of relative position, orientation, size and shape of cartilage elements. Although the process of cartilage histogenesis appears generally conserved across vertebrates (Eames et al., 2007), cartilage morphogenesis has achieved remarkable evolutionary diversification. We analyzed histogenesis and morphogenesis of Meckel’s cartilage in the lower jaws of quail, duck and quck chimeras, and focused on the acquisition of size and shape. Meckel’s cartilage of the quail is much smaller than that of a stage-matched duck and becomes distinctly shaped over time. Moreover, quail embryos develop at a significantly quicker rate than do duck embryos. Such spatiotemporal differences allow chimeric quck embryos to uncover mesenchyme-dependent aspects of histogenesis and morphogenesis. Our results demonstrate that mesenchyme determines both stage-specific and species-specific size and shape, and does so by exerting spatiotemporal control over the molecular and histogenic programs for cartilage. These findings shed light on cellular mechanisms and signaling interactions regulating skeletal pattern, the functioning of developmental modules underlying chondrogenesis, and the role of heterochrony during the evolution of species-specific size and shape.
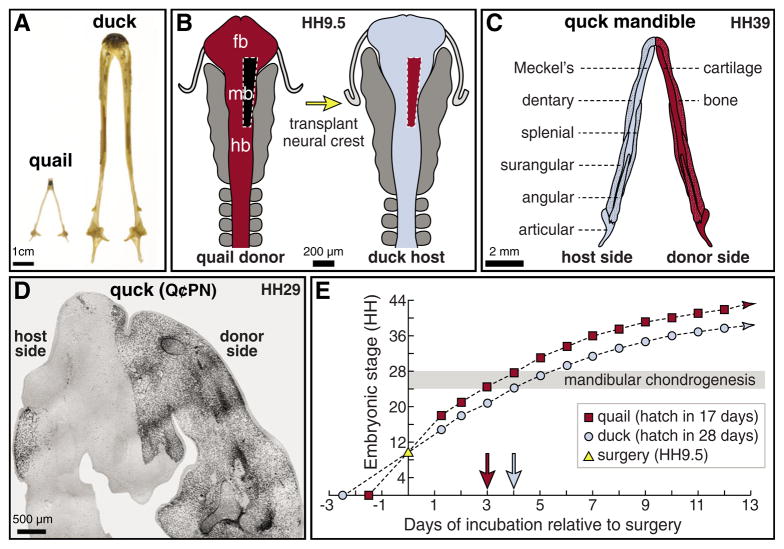
Fig. 1. Experimental design and methods.
(A) Lower jaw skeletons of adult Japanese quail (Coturnix coturnix japonica) and white Pekin duck (Anas platyrhyncos). (B) Schematic of rostral neural tube at HH9.5, depicting the levels of neural crest cells grafted from quail to duck. (C) Schematic of a lower jaw skeleton at HH39, depicting the contributions of transplanted neural crest (red) to cartilage and bone (stippled). (D) Horizontal section through the mandibular primordium of a HH29 chimeric quck embryo (rostral at top), which will give rise to the lower jaw skeleton. Quail donor mesenchyme (black), as visualized by the quail-specific antibody Q¢PN, was found throughout the transplanted side, whereas few to no quail cells were observed on the contralateral duck host side. (E) Graph illustrating the distinct developmental trajectories of quail (red squares) versus duck (blue circles) after being stage-matched at HH9.5 for surgery (yellow triangle on y-axis). Control quail and duck embryos were separated by approximately three HH stages within 2 days of surgery, and throughout the initial stages of overt mandibular chondrogenesis (gray area).
MATERIALS AND METHODS
Generation of chimeric embryos
Fertilized eggs of Japanese quail (Coturnix coturnix japonica) and white Pekin duck (Anas platyrhynchos) were purchased from AA Labs (Westminster, CA) and incubated at 37°C. Embryos were handled following University and NIH guidelines. Embryos were matched at stage 9.5 using the Hamburger and Hamilton (HH) system for chicks (Hamburger and Hamilton, 1951) applied to quail (Le Douarin et al., 1996) and duck (Yamashita and Sohal, 1987; Eames and Schneider, 2005; Lwigale and Schneider, 2008). At HH9.5, neural crest cells are abundant along the dorsal midline of the rostral neural tube (Tosney, 1982). Either unilateral or bilateral populations of neural crest cells generated by the caudal forebrain, midbrain and rostral hindbrain were grafted orthotopically from quail to duck (Fig. 1B). Tungsten needles and Spemann pipettes were used for surgical operations (Hamburger, 1942; Schneider, 1999). Donor graft tissue was positioned and inserted into a host that had an equivalent region of tissue excised. Control orthotopic grafts and sham operations were made within each species. Controls were incubated alongside chimeras to ensure that stages of grafted cells were assessed accurately.
Histology and immunohistochemistry
Control and chimeric embryos were fixed in Serra’s (100% ethanol:37% formaldehyde:glacial acetic acid, 6:3:1) overnight at 4°C. Embryos were paraffin embedded and cut into 7 μm sections. Some sections were stained following the Hall Brunt quadruple (HBQ) method (Hall, 1986) for histological visualization of cartilage. To detect donor cells in chimeric embryos, adjacent sections were immunostained with the quail nuclei-specific Q¢PN antibody [1:10, Developmental Studies Hybridoma Bank (DSHB)] (Schneider, 1999). Immunostaining for collagen type 2 (1:25; DSHB antibody II-II6B3) was carried out similarly, except sections underwent microwave-induced epitope retrieval in 0.01 M citrate buffer, and enzymatic digestion with Ficin (Zymed: South San Francisco, CA). Sections were imaged using brightfield or differential interference contrast.
Whole embryos were stained with Alcian Blue and Alizarin Red (Wassersug, 1976). For early stages, embryos were stained overnight in 0.1% Alcian Blue (in 70% ethanol, 30% glacial acetic acid), rehydrated, washed with 0.5% potassium hydroxide and cleared with glycerol.
Gene expression analyses
In situ hybridization was performed (Albrecht et al., 1997). Sections adjacent to those used for histological and immunohistochemical analyses were hybridized with 35S-labeled chick riboprobes to sox9 (transcription factor), col2a1 (fibrillar collagen), fgf4 and fgf8 (secreted ligands), and fgfr2 (receptor). These chick probes specifically and equivalently identified counterparts in quail and duck tissue (data not shown). Sections were counterstained with Hoechst dye (Sigma). Hybridization signals were detected using darkfield and the nuclear stain with epifluorescence.
Morphometric analyses
To quantify changes in size and shape of Meckel’s cartilage that occurred after unilateral transplantation of neural crest, a landmark-based two-dimensional morphometric analysis was performed (Coppinger and Schneider, 1995; Schneider and Helms, 2003). Whole-mount preparations of control and chimeric mandibles were imaged at the same magnification. Specimens were aligned in a consistent orientation. The X and Y axes were set at zero along the planes passing through the distal tip. Fifteen landmarks were selected along the perimeter of Meckel’s cartilage based on anatomical boundaries, extrema and midpoints (Zelditch, 2004). Landmarks were: (1) distal tip of Meckel’s; (2) medial maximum distal width; (3) lateral maximum distal width; (4) proximal tip of articular; (5) medial maximum proximal width of articular tip; (6) lateral maximum proximal width of articular tip; (7) medial maximum width of articular; (8) lateral junction between Meckel’s and articular; (9) medial junction between Meckel’s and articular; (10) medial midpoint between distal and proximal tips; (11) lateral midpoint between distal and proximal tips; (12) medial midpoint between midpoint and distal tip; (13) lateral midpoint between midpoint and distal tip; (14) medial midpoint between midpoint and junction between Meckel’s and articular; (15) lateral midpoint between midpoint and junction between Meckel’s and articular.
Coordinate data for each landmark were obtained using the information tool in Photoshop and inputted into the Paleontological Statistics Software Package for Education and Data Analysis (PAST) (Hammer and Harper, 2006). Specimens were averaged within groups and analyzed using a Procrustes algorithm, which processes sets of X, Y coordinates, removes the factor of size and reveals shape changes (Chapman, 1990). The average of the squared magnitudes of the vectors produced distance coefficients that were used in cluster analyses (Ward’s method).
FGF signaling inhibition in mandibular explants
To test the ability of FGF signaling to regulate the timing of chondrogenesis, quail mandibular primordia were dissected at HH24, placed on Transwell membranes (0.4 μm pore size, Corning), and immersed in minimal BGJb medium (Merrill et al., 2008). The FGF receptor inhibitor SU5402 (Calbiochem) (25 μM) was dissolved in DMSO and added to the media either at the time of culture or 24 hours later. Controls were treated with DMSO alone. Dose was based on a previous study (Mandler and Neubuser, 2004). Mandibles were cultured for up to 3 days and processed for histology and immunohistochemistry.
RESULTS
Mesenchyme regulates cartilage size and shape
To investigate the ability of mesenchyme to generate cartilage size and shape, we transplanted unilateral pre-migratory populations of neural crest cells between stage-matched quail and duck embryos (Fig. 1B). These quail donor cells ultimately filled the right half of the duck host mandible, as confirmed by immunostaining with the quail-specific antibody Q¢PN (Fig. 1C,D). This experimental approach maintained the non-surgical side as an internal control (Tucker and Lumsden, 2004; Eames and Schneider, 2005), and allowed for a clear comparison of quail donor- and duck host-derived Meckel’s cartilage in the same chimeric mandible. An additional analytical tool was the considerable difference in growth rate between quail and duck; within 2 days of surgery and throughout mandibular chondrogenesis, quail and duck embryos were separated by three HH stages (Fig. 1E).
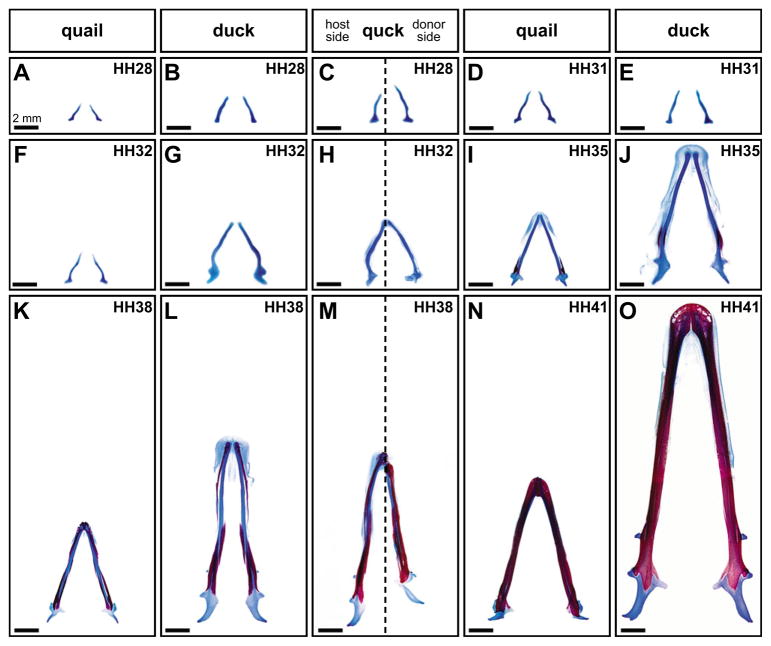
Analysis of Meckel’s cartilage in control embryos revealed that quail and duck exhibit stage-specific and species-specific differences in size and shape. During early embryonic stages (HH28-32), Meckel’s cartilage of quail and duck transitioned from a slightly curved morphology to an S-shaped lateral bend morphology (Fig. 2A,B,D–G). By HH35, Meckel’s cartilage in quail acquired a relatively straight morphology (Fig. 2I,J) whereas duck maintained more curvature. Meckel’s cartilage in quail and duck grew in each successive stage from HH28-41, but duck cartilage elements were consistently larger (e.g. compare Fig. 2F with 2G), reflective of their final adult morphology (Fig. 1A). In HH28 quck chimeras, Meckel’s cartilage on the quail donor-derived side appeared longer than Meckel’s cartilage on the contralateral duck host side, and was similar to that of HH31 control quail (n=6; Fig. 2C). This is consistent with the observation that HH31 quail Meckel’s cartilage is slightly longer than HH28 duck Meckel’s cartilage (compare Fig. 2D with 2B). In HH32 quck mandibles, the duck host-derived Meckel’s cartilage was the same size and shape as in duck controls, but the quail-derived Meckel’s cartilage exhibited a more straightened morphology like that observed in quail controls at HH35 (Fig. 2H,I). At HH38, the quail-derived Meckel’s cartilage in quck mandibles was morphologically distinct from the contralateral element of duck origin, and approximated the size and shape of HH41 quail control Meckel’s cartilage (n=4; Fig. 2M). Thus, quail donor mesenchyme maintained its faster rate of development within the relatively slower duck host environment, and Meckel’s cartilage on the donor side was consistently more advanced in terms of size and shape than that observed on the contralateral host side.
Fig. 2. Mesenchyme determines the size and shape of Meckel’s cartilage.
(A,B) Meckel’s cartilage in control quail and duck embryos was relatively short at HH28 (stained with Alcian Blue and shown in ventral view with distal towards the top). (C) In HH28 chimeric quck mandibles, the host Meckel’s cartilage was equivalent to an HH28 duck, but the quail donor side (right of broken line) resembled the size and shape of a control quail Meckel’s cartilage at HH31. (D,E) Meckel’s cartilage was slightly curved at HH31 in quail and duck. (F,G) At HH32, Meckel’s cartilage was S-shaped in quail and duck. (H) In HH32 quck, the host Meckel’s cartilage was like an HH32 control duck, but the quail donor side matched the size and shape of a quail Meckel’s cartilage at HH35. (I,J) By HH35, Meckel’s cartilage began to straighten, but some curvature persisted in duck. (K,L) This straightened morphology became augmented by HH38. (M) In HH38 quck mandibles, both the quail-derived Meckel’s cartilage and the contralateral duck Meckel’s cartilage were straightened, but the quail-derived Meckel’s cartilage was shorter than its duck-derived counterpart, and was more similar in size to control quail Meckel’s cartilage at HH41. (N,O) By HH41, the size and shape of Meckel’s cartilage was reflective of adult morphology.
To control for the possibility that the observed size and shape changes to Meckel’s cartilage were due to local mechanical forces such as tension at the chimeric midline from asymmetric muscle attachments, we also performed bilateral transplants. In all of the resultant bilateral chimeric quck, Meckel’s cartilage was transformed on both sides in a manner equivalent to that observed only on one side in unilateral chimeric quck (n=7; data not shown). Thus, the morphological transformations are due entirely to quail donor-mediated programs for cartilage size and shape.
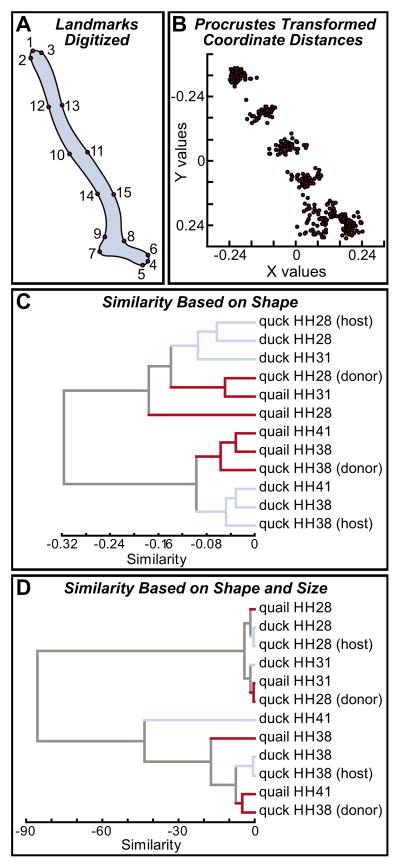
We quantified changes in Meckel’s cartilage morphology by employing a landmark-based morphometric approach (Coppinger and Schneider, 1995; Schneider and Helms, 2003). Landmark points defining Meckel’s cartilage were evaluated using a Procrustes algorithm (Fig. 3A,B), which outputs distance coefficients that summarize differences from pair-wise comparisons between all specimens. These distance coefficients were used in cluster analyses to reveal trends in the morphometric data. Based on overall similarity in the shape of Meckel’s cartilage, quail cluster with the donor sides of quck embryos, and are distinct from the group that includes duck and the host sides of quck (Fig. 3C). To assess the contributions of stage-specific and species-specific differences in size, we also included centroid values in the distance matrices and performed another cluster analysis (Fig. 3D). In this case, the groups break down primarily on the basis of embryonic stage, rather than species, so that quail, duck and the host side of quck at HH28 cluster together; HH31 quail, HH31 duck and the donor side of HH28 quck cluster together; the HH38 and HH41 specimens cluster together with the duck and host side of quck at HH38 forming one group and the HH41 quail and donor side of quck at HH38 forming another. Overall, our experiments demonstrate that the stage-specific and species-specific size and shape of Meckel’s cartilage are established as a function of the neural crest-derived mesenchyme.
Fig. 3. Landmark-based analysis of ontogenetic and phylogenetic size and shape.
(A) Fifteen landmark points were selected along Meckel’s cartilage in quail, duck and/or quck embryos at HH28, HH31, HH38 and HH41. (B) X, Y coordinate data were analyzed using a Procrustes method, which removes the factor of size and reveals shape differences. (C) The average of the squared magnitudes of the vectors produced distance coefficients that were used in cluster analyses (unweighted pair group method using arithmetic averages). On the basis of overall shape similarity, duck at HH28 and HH31, and the duck host side of quck at HH28 were more alike than quail at HH31 and the quail donor side of quck at HH28; quail at HH38 and HH41, and the quail donor side of quck at HH38 were more alike than duck at HH38 and HH41, and the duck host side of quck at HH38. (D) When differences in size were included in the analysis, the groups clustered mostly by stage rather than by species. In addition, the relative amount of similarity was much less between early and late stages due to the vast differences in size between early and late stages (i.e. those associated with growth), and between quail and duck (i.e. those that are species specific).
Mesenchyme controls the initiation of overt chondrification
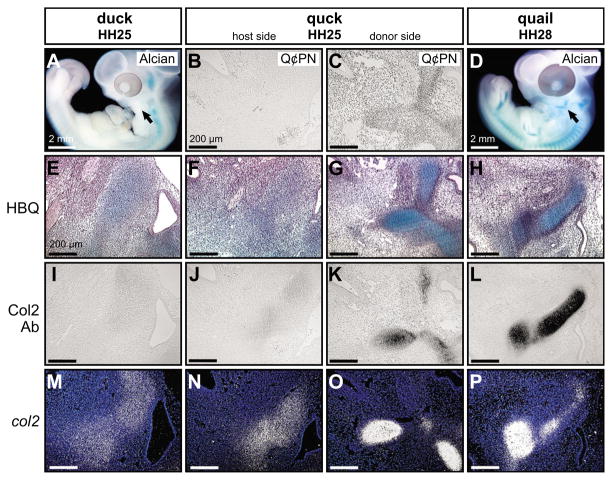
To identify a mechanism through which neural crest mesenchyme carries out its morphogenetic program for size and shape, we focused on the process of histogenesis. We investigated the extent to which histogenic programs of chondrogenesis were altered in chimeric mandibles. We collected quck at stages that could potentially reveal spatiotemporal shifts in overt chondrification, which is when chondroblasts begin to secrete cartilaginous matrix (Hall, 2005). We assayed for changes in overt chondrification of Meckel’s cartilage and the adjacent quadrate cartilage. As visualized by Alcian Blue staining of whole-mount control duck and quail embryos, the quadrate and Meckel’s cartilage were not detected at HH25, but became conspicuous by HH28 (Fig. 4A,D; see also Fig. 5D). A light and diffuse Alcian Blue stain was observed in sections through the proximolateral aspect of mandibles in HH25 duck and quail embryos (Fig. 4E; see also Fig. 5H). No Col2-immunoreactivity was apparent in chondrifying regions of control HH25 duck and quail mandibles (Fig. 4I). Expression patterns for transcripts of col2a1 were in similar domains as the light Alcian Blue staining of HH25 duck and quail control mandibles (Fig. 4M; see also Fig. 5L,P). However, there were no distinct tissue boundaries, such as a perichondrium, separating col2a1-positive cells from surrounding mesenchyme.
Fig. 4. Mesenchyme regulates late histogenesis of Meckel’s cartilage.
(A) Whole-mount Alcian Blue stained embryos at HH25 reveal that cartilage has yet to form in proximo-lateral regions of the avian mandible (arrow). (B) Duck host mesenchyme was negative for the anti-quail antibody Q¢PN, as shown in sagittal section. (C) By contrast, donor sides of HH25 chimeric quck mandibles contained abundant quail neural crest-derived mesenchyme. (D) Jaw cartilages became obvious by HH28 (arrow). (E) HBQ-stained histological sections through the jaw joint of control embryos at HH25 revealed diffuse Alcian Blue staining in mesenchyme with ill-defined borders. (F) Similar low diffuse levels of Alcian Blue were observed in host sides of HH25 quck mandibles. (G) In conjunction with the presence of relatively older quail donor cells, developing cartilages of quck chimeras stained strongly with Alcian Blue and exhibited a defined perichondrium. (H) Robust Alcian Blue staining and a clear perichondrium characterized developing cartilages at HH28. (I) Mesenchyme of the mandible was not immunoreactive for Collagen type II protein (Col2) in control embryos at HH25. (J) The host sides of chimeras were also negative for Col2 protein. (K) Quail-derived mesenchyme of HH25 quck mandibles demonstrated strong Col2-immunoreactivity. (L) Control HH28 mandibular cartilages were also positive for Col2 protein. (M) col2a1 expression appeared diffuse in developing mandibular cartilages in control HH25 embryos. (N) The host side of quck at HH25 also showed low levels of col2a1 expression. (O) Quail-derived mesenchyme of HH25 chimeric mandibles had more spatially resolved col2a1 domains, as well as higher col2a1 expression levels, when compared with contralateral duck host mesenchyme. (P) Similar expression domains were observed at HH28 in control quail.
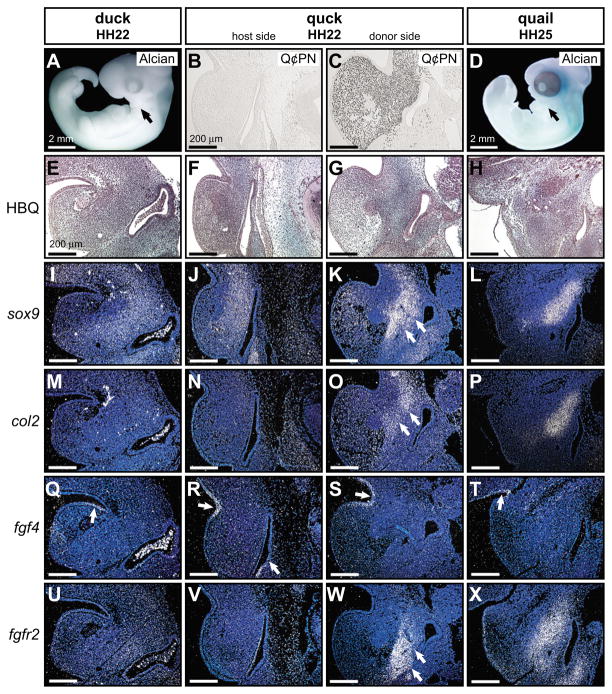
Fig. 5. Mesenchyme regulates early histogenesis of Meckel’s cartilage.
(A) Whole-mount Alcian Blue stained embryos at HH22 reveal that cartilage has yet to form in proximo-lateral regions of the avian mandible (arrow). (B) Duck host mesenchyme was negative for the anti-quail antibody Q¢PN as shown in sagittal section. (C) By contrast, donor sides of HH22 chimeric quck mandibles contained abundant quail neural crest-derived mesenchyme. (D) Jaw cartilages were still not present at HH25 (arrow). (E–H) Similarly, HBQ-stained histological sections through the jaw joint of control and chimeric embryos revealed diffuse Alcian Blue staining in mandibular mesenchyme. (I,J) The chondrogenic transcription factor sox9 was expressed broadly at low levels from the endodermal pouch across mandibular mesenchyme in control embryos, as well as the host side of chimeric quck at HH22. (K) On the donor side, coincident with Q¢PN-positive mesenchyme, sox9 expression was restricted at a distance from the endodermal pouch and levels were considerably higher than that observed on the contralateral host side. (L) Expression of sox9 in control quail embryos at HH25 was equivalent to the donor side of quck at HH22. (M,N) col2a1 was not detected in mandibular mesenchyme of control embryos or the host side of quck at HH22. (O,P) However, col2a1 was expressed in the pre-cartilaginous condensations on the donor side of quck and of control embryos at HH25. (Q–T) fgf4 was expressed continuously at HH25, HH22 and earlier in the epithelium of control and chimeric mandibles. (U,V) fgfr2, which encodes a receptor for FGF4, was not expressed in mandibular mesenchyme of control embryos or in the host side of chimeric quck at HH22. (W,X) fgfr2 transcripts were abundant in quail donor-derived mesenchyme of quck at HH22 like that observed in controls at HH25.
Upon analysis of the same region in HH28 control quail and duck mandibles, many features of overt chondrification were apparent. Alcian Blue staining was much stronger in both quail and duck than at HH25 (e.g. compare Fig. 4H with 4E) and the matrix was surrounded by a distinct perichondrium (Fig. 4H). Col2-positive matrix was detected in the quadrate and Meckel’s cartilage of HH28 control duck or quail embryos (Fig. 4L). Expression domains of col2a1 were more spatially defined and the levels appeared much higher in chondrifying regions of HH28 control mandibles than in similar regions of HH25 control embryos for both quail and duck (compare Fig. 4P with 4M).
Q¢PN antibody staining on sections through proximolateral regions of HH25 chimeric quck mandibles confirmed an abundance of quail donor-derived mesenchyme on the transplanted sides (Fig. 4B,C). In HH25 quck mandibles, developing cartilages from quail donor mesenchyme exhibited strong Alcian Blue staining, as well as a distinct perichondrial boundary, which was unlike that observed in contralateral duck host mesenchyme (Fig. 4F,G). Additionally, only the donor side exhibited Col2 immunoreactivity (Fig. 4J,K) and the domains and expression levels of col2a1 in quail donor-derived mesenchyme of HH25 quck mandibles appeared more similar to control quail tissues at HH28 than to the more diffuse domains observed in contralateral HH25 duck host mesenchyme (Fig. 4N–P). Therefore, quck chimeras revealed that neural crest-derived mesenchyme autonomously expresses histological and molecular markers of overt chondrification.
Mesenchyme controls the initiation of chondrogenic condensation
To examine the extent to which neural crest mesenchyme regulates the earliest stages of chondrogenesis, we analyzed molecular markers expressed during the formation of chondrogenic condensations. As visualized histologically in HH22 control duck and quail mandibles, a very light and diffuse Alcian Blue stain was observed in mesenchymal cells located deep to the surface ectoderm and abutting the endodermal pouch of the pharynx (Fig. 5E). In the same region of control duck and quail embryos at HH25, larger condensations of cells became more conspicuous histologically, although no distinct perichondrium was observed (Fig. 5H; see also Fig. 4H). Although there were low levels of sox9 transcripts in mandibular mesenchyme of control duck and quail at HH22 (Fig. 4I), the spatial domain of sox9 expression underwent a restriction by HH25 that reflected histological observations of a chondrogenic condensation (Fig. 4L; Fig. 3E). Moreover, a clear spatial separation was apparent between sox9-expressing cells and the endodermal pouch at HH25, whereas no such separation was observed at HH22 (Fig. 5I,L). Furthermore, in both control duck and quail mandibles at HH22, no transcripts for col2a1 were detected but they appeared in chondrogenic condensations by HH25 (Fig. 5M,P; see also Fig. 4M). Finally, to assay for changes in signaling by growth factors known to be associated with cartilage formation, we analyzed expression of fgf4, fgf8 and the receptor fgfr2. We observed epithelial expression of fgf4 and fgf8 in quail and duck embryos at HH22 and HH25 (Fig. 5Q,T; and data not shown), which is consistent with published reports that several fgf genes are expressed in mandibular epithelium as early as HH15 and thereafter (Wall and Hogan, 1995; Shigetani et al., 2000; Mina et al., 2002; Havens et al., 2006). Although we did not detect transcripts for fgfr2 in the mandible at HH22, we did in the mesenchyme at HH25 (compare Fig. 5U to 5X).
Q¢PN antibody staining of HH22 chimeric mandibles confirmed an abundance of quail donor neural crest-derived mesenchyme on transplanted sides, compared with the contralateral duck host side (Fig. 5B,C). The quail donor side of HH22 quck mandibles exhibited a more robust condensation of cells, which also expressed sox9, when compared with the contralateral duck host side (Fig. 5F,G,J,K). The distinct spatial separation of the sox9-expressing cells from the endodermal pouch on the quail donor side was more similar to that observed in HH25 control quail embryos than that seen in the contralateral duck host side, or in control duck embryos at HH22. In addition, quail neural crest-derived mesenchyme of HH22 quck mandibles expressed col2a1 and fgfr2 in chondrogenic condensations, whereas no transcripts could be detected in contralateral duck mandibular mesenchyme (Fig. 5N,O,V,W).
FGF signaling regulates the timing of mandibular chondrogenesis
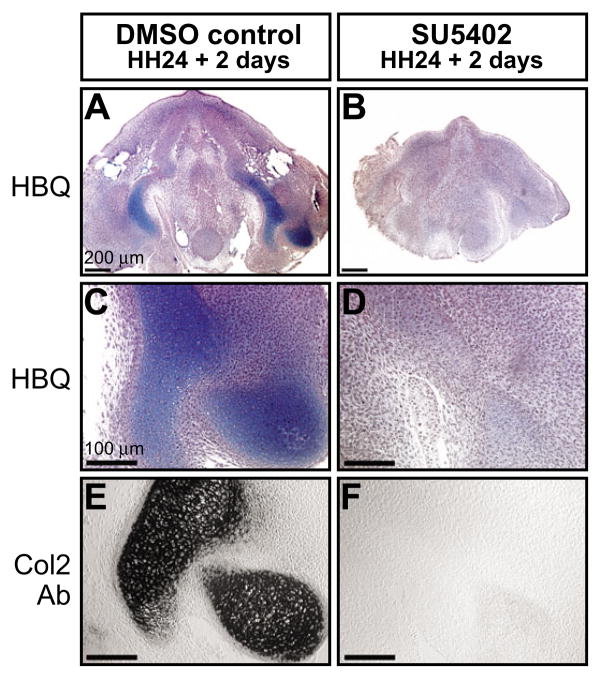
To determine the extent to which the upregulation of fgfr2 in quail donor mesenchyme is mechanistically related to premature cartilage formation, we performed experiments designed to test the ability of FGF signaling to regulate the timing of mandibular chondrogenesis. We treated quail mandibles with SU5402, which inhibits the tyrosine kinase activity of fibroblast growth factor receptors (Mohammadi et al., 1997). Our treatments exposed quail neural crest mesenchyme to SU5402 immediately prior to when fgfr2 normally becomes upregulated in mandibular mesenchyme. We found that an inhibition of FGF signaling at this precise time causes a significant delay in chondrogenesis (n=5; Fig. 6), whereas treating 24 hours later has little effect (n=5; data not shown). Additional experiments revealed that chondrogenesis was delayed rather than blocked entirely by SU5402, as culturing treated mandibles for an additional day enabled cartilage to eventually form (n=5; data not shown). Thus, temporal changes to FGF signaling either through biochemical manipulation or via the quail-duck chimeric system can alter the timing of cartilage formation.
Fig. 6. FGF signaling regulates the timing of mandibular chondrogenesis.
(A,C) Quail mandibles harvested at HH24 and cultured for 2 days show robust histological staining throughout Meckel’s cartilage (Alcian Blue). (B,D) Those mandibles treated biochemically with SU5402, which inhibits FGF signaling, lack cartilage matrix staining. (E) Collagen type II protein (Col2) is detected in control mandibles after 2 days of culture. (F) No Col2 protein is observed following treatment with SU5402.
DISCUSSION
Mesenchyme controls mandibular chondrogenesis
The question of how complex anatomical systems acquire their proper three-dimensional morphology, has concerned biologists for almost a century. In his 1928 essay ‘On Being the Right Size’, J. B. S. Haldane remarked that ‘The most obvious differences between different animals are differences of size…For every type of animal there is a most convenient size, and a large change in size inevitably carries with it a change in form’ (Haldane, 1985). Using the quail-duck chimeric system, our morphometric and molecular analyses of Meckel’s cartilage reveal that both stage-specific and species-specific size and shape arise from the neural crest-derived mesenchyme.
To understand developmental mechanisms through which mesenchyme controls cartilage size and shape, we identified changes in the program of cartilage histogenesis that preceded changes in morphogenesis. Chondroblasts on the quail donor side of quck mandibles differentiated on the timeframe of quail controls as opposed to that of the contralateral duck host. Donor-dependent shifts in cartilage histogenesis were apparent from the beginning of mesenchymal condensation. Both sox9, which is the earliest known molecular marker of chondrogenic condensations (Healy et al., 1996; Zhao et al., 1997; Eames et al., 2003; Eames et al., 2004), and col2a1, which is directly regulated by sox9 (Bell et al., 1997), were expressed prematurely by quail donor cells relative to duck host mesenchyme of the contralateral side. Moreover, we found that FGF signaling, which functions upstream of sox9 and chondrogenesis (Healy et al., 1999; de Crombrugghe et al., 2000; Murakami et al., 2000; Petiot et al., 2002; Eames et al., 2004; Govindarajan and Overbeek, 2006; Bobick et al., 2007) is also regulated by mandibular mesenchyme. To test the mechanistic significance of these results, we inhibited FGF receptor activation and discovered that, during a discrete temporal window, FGF signaling plays a role in setting the timing of mandibular chondrogenesis. Thus, by regulating the timing of FGF signaling as well as the expression of sox9 and col2a1, mandibular mesenchyme likely transmits information for stage-specific and species-specific size and shape to Meckel’s cartilage.
What role do epithelia play during chondrogenesis?
Although our experiments reveal that cartilage development involves mesenchymally mediated histogenesis and morphogenesis, numerous studies have also demonstrated important roles for adjacent epithelia. For example, the ‘flypaper model’ advanced the concept that epithelial-mesenchymal interactions produce an abundant extracellular matrix, which adhesively traps migrating neural crest cells at their site of differentiation and leads to the induction of cartilage (Garrod, 1986; Thorogood, 1988; Thorogood, 1993). In the head, such epithelia are derived from the surface ectoderm, brain, sensory capsules and pharyngeal endoderm, and also seem to be required for the initiation and maintenance of chondrogenesis (Hall, 1980; Hall, 1981; Thorogood et al., 1986), although some evidence suggests that epithelia may also inhibit chondrogenesis (Mina et al., 1994). Many studies have shown that epithelia provide axial information to the underlying mesenchyme. For example ectopic rotation of facial ectoderm induces mirror image duplications of distal upper beak structures along the dorsoventral axis (Marcucio et al., 2005). Additionally, pharyngeal epithelium conveys region-specific polarity and segmental identity to neural crest mesenchyme, as demonstrated by transplantation studies (Couly et al., 2002) and genetic analyses (Kimmel et al., 1998; Veitch et al., 1999; Miller et al., 2000; Piotrowski and Nusslein-Volhard, 2000; Kikuchi et al., 2001; David et al., 2002; Crump et al., 2004).
The revelation that mesenchyme drives significant aspects of cranial chondrogenesis can be reconciled with known functions of epithelia, following a relatively straightforward scenario. In this study, we have focused primarily on mesenchymal condensation, overt chondrocyte differentiation and cartilage morphogenesis. The roles of pharyngeal endoderm and facial ectoderm, for example, in establishing cartilage orientation and regional identity around the oral cavity may occur prior to mesenchymal condensation, perhaps by permitting or aligning the spatial distribution of cartilage condensations in mandibular mesenchyme. In this sense, these epithelia would be acting instructively at first but then assume a more permissive role that enables the execution of mesenchyme-dependent programs and allows chondrogenesis to progress in a somewhat time-independent manner. Thus, although epithelia derived from the endoderm and ectoderm may determine the location of chondrogenic condensations, which does not appear to differ grossly between quail and duck, our data show that neural crest-derived mesenchyme subsequently responds through intrinsic, stage-specific and species-specific programs of histogenesis and morphogenesis that ultimately regulate cartilage size and shape. Such findings gain support from the similar permissive role played by epithelia during mandibular osteogenesis (Merrill et al., 2008), and the observation that many chondrogenic signals including FGFs and BMPs are continuously expressed by epithelia prior to and during the arrival of neural crest-derived mesenchyme in the mandible (Francis-West et al., 1994; Wall and Hogan, 1995; Shigetani et al., 2000; Ashique et al., 2002; Mina et al., 2002; Havens et al., 2006).
Mesenchyme integrates programs of cartilage histogenesis and morphogenesis
Despite being conceptually distinct, histogenesis and morphogenesis appear so tightly coupled during mandibular chondrogenesis that they function as a single developmental module. Such results confirm other studies in zebrafish, where early morphogenesis of cells in pharyngeal cartilages is coincident with histogenesis (Kimmel et al., 1998), and sox9−/− mutants have defects in both cartilage morphogenesis and histogenesis (Yan et al., 2002). Runx2−/− mice also illustrate the interplay between histogenesis and morphogenesis, for the absence of chondrocyte hypertrophy leads to a loss of cartilage growth (Yoshida et al., 2002; Iwamoto et al., 2003). In a similar vein, our quck studies could not dissociate morphogenesis from histogenesis. Morphogenetic aspects of quck cartilage development, such as size and shape acquisition, were altered temporally to the same extent as histogenic features, such as secretion of extracellular matrix. Although the possibility exists that additional events during histogenesis and morphogenesis, including the specification of pre-chondrogenic mesenchyme or spatial initiation of condensations, occur independently of mesenchyme, we think this is unlikely as all aspects of chondrogenesis that we examined (e.g. matrix deposition, protein synthesis and gene expression), were altered in our experimental system.
Thus, our results support the notion that histogenesis and morphogenesis are highly inter-dependent processes, and that their integration during chondrogenesis characterizes a developmental module defined primarily by the autonomy of neural crest-derived mesenchyme (Fig. 7). This is similar to the role proposed for mesenchyme in other developmental modules such as the formation of epidermal appendages (Eames and Schneider, 2005; Schneider, 2005). The modularity of chondrogenesis makes sense given that cartilage morphogenesis relies on histogenesis to generate proper three-dimensional form, and many of the same molecules and signaling pathways that function during early neural crest cell specification, proliferation and differentiation, such as BMPs and FGFs, are also known to affect later cartilage pattern (i.e. size and shape) in the avian oral cavity (Francis-West et al., 1994; Mina et al., 1995; Wall and Hogan, 1995; Barlow and Francis-West, 1997; Ekanayake and Hall, 1997; Richman et al., 1997; Barlow et al., 1999; Tucker et al., 1999; Wang et al., 1999; Shigetani et al., 2000; Ashique et al., 2002; Mina et al., 2002; Abzhanov et al., 2004; Wilson and Tucker, 2004; Havens et al., 2006; Schneider, 2007). But BMPs and FGFs also appear to function divergently, especially given their effects on upper versus lower regions of the beak, the times at which they act and the tissues in which they are expressed. For example, published work on the ability of BMPs to regulate size and shape pertain primarily to the upper beak, and, in particular, to the frontonasal process (Abzhanov and Tabin, 2004; Wu et al., 2004; Wu et al., 2006; Foppiano et al., 2007). Moreover, our prior experiments show that BMP signaling acts slightly later during mandibular development to regulate the timing of bone formation, but not of cartilage (Merrill et al., 2008).
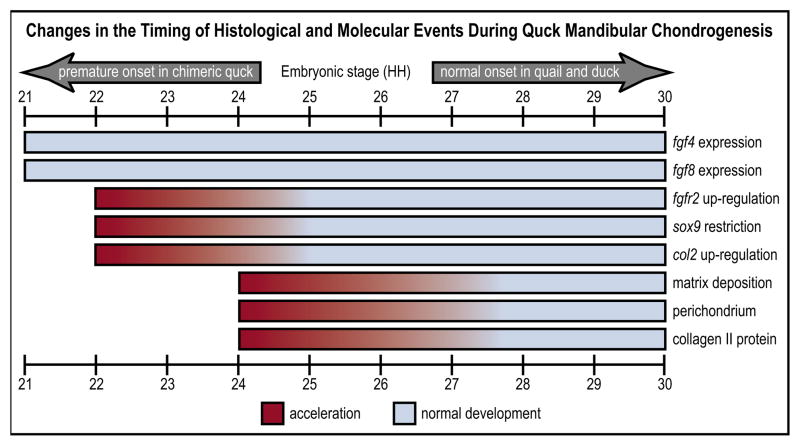
Fig. 7. Mesenchymal regulation of chondrogenesis.
Quail-duck chimeras reveal spatiotemporal plasticity in the molecular and histogenic programs underlying cartilage development. Bars represent stages when events are initiated in quail and duck, and the extent to which they are accelerated in quck chimeras.
Quail-duck chimeras test for the effects of heterochronies on size and shape
From the perspective of evolutionary developmental biology, one off the most striking findings from this work is the ability of mesenchyme to keep track of stage-specific and species-specific size and shape concurrently. Presumably, quail donor mesenchyme accomplishes this task, in part, by shifting the timing of histogenic events in the duck to something like that found in the quail. This result provides a novel mechanism for linking skeletal development and evolution. One prominent concept that has been used previously to connect development with evolution, and to study transformations in size and shape, is heterochrony. Traditionally, heterochrony describes changes in the timing of developmental events between an ancestor and a descendant (Russell, 1916; de Beer, 1930). Clearly quail and duck do not have an ancestor-descendant relationship, but heterochrony also applies to comparisons of closely related taxa, and in this regard can be used to evaluate the effects of variations in rates of growth on size and shape (Gould, 1977; Alberch et al., 1979; Hall, 1984; Roth, 1984; Foster and Kaesler, 1988; McKinney, 1988; Klingenberg and Spence, 1993; Raff, 1996). Such growth heterochrony is just one variable introduced by the faster developing quail donor cells in a relatively slower duck host (i.e. manifest through intrinsic species-specific differences in maturation rates). Another variable in this chimeric system would be the experimentally induced shifts in the relative onsets, cessations and/or durations of molecular and cellular events during histogenesis. In naturally occurring systems, such changes have been termed sequence heterochrony, and can be used to analyze the evolution of size and shape (Smith, 2001; Smith, 2002; Smith, 2003), especially in the context of reciprocal epithelial-mesenchymal interactions underlying skeletal diversity (Smith and Hall, 1990).
Our results reveal that quail donor mesenchyme carries information on the rate and time at which chondrogenesis should proceed. Most likely, this predisposition arises from intrinsic mechanisms that control the cycling and proliferation of cells in a quail-specific manner. The consequence is that chondrogenesis advances by three embryonic stages, which is similar to what we observed in during feather morphogenesis and osteogenesis (Eames and Schneider, 2005; Merrill et al., 2008), and Meckel’s cartilage attains species-specific size and shape. Other possible outcomes could have been that quail donor cells follow the timetable of the host and make cartilage that is either quail-like or duck-like in morphology; or they generate some novel anatomy that is either a combination of, or unlike that normally observed in quail or duck. Instead, we find that quail donor mesenchyme introduces a significant alteration in the timing and rates of histogenic events and executes a program of cartilage morphogenesis like that normally observed in quail. As such, our results suggest that heterochronic changes can generate species-specific morphology, but they do so with at least two very conspicuous caveats. First, in terms of absolute time, there really is no heterochrony as quail donor cells followed their own schedule and behaved as quail cells normally do. The heterochrony we introduced can only be considered in terms of relative timing (i.e. to that of the duck host) of molecular and cellular events during chondrogenesis. However, such sequence heterochrony may not have occurred if quail cells were able to accelerate all relevant duck host events immediately after neural crest transplantation. Second, quail-specific morphology was achieved not merely by an acceleration of developmental events but also by progressive implementation of a quail-specific genome. In this capacity, quail donor mesenchyme may simply be responding to common signals present in duck host epithelium that are continuously expressed during a broad developmental window (e.g. fgf4), and which are able to accommodate the difference in stage between donor-derived and host-derived mesenchyme. Studies that employ species with wider disparities in growth rates could resolve this possibility by elucidating the limits of competency in either the donor or host, although our published data already confirm that epithelium can respond to premature mesenchymal induction (Schneider and Helms, 2003; Eames and Schneider, 2005). Overall, the remarkable propensity of neural crest-derived mesenchyme to impart size and shape across embryonic stages and between species in parallel, points to the generative role that development has played during the course of morphological evolution.
Acknowledgments
We thank Kristin Butcher, Johanna Staudinger, Christian Solem and Nicole Grady for technical assistance; Andrew Jheon, Amy Merrill, Christian Mitgutsch, Masayoshi Tokita, Nathan Young and Kathleen Smith for helpful discussions; and Thomas Dam at AA Lab Eggs. The Q¢PN and II-II6B3 antibodies were obtained from the DSHB, maintained by the University of Iowa under the auspices of the NICHD. Funded in part, by an NIH F32 DE016778 to B.F.E.; and by R03 DE014795 and R01 DE016402 from the NIDCR, R21 AR052513-01 from the NIAMS, and Research Grant 5-FY04-26 from the March of Dimes Birth Defects Foundation to R.A.S.
References
- Abzhanov A, Tabin CJ. Shh and Fgf8 act synergistically to drive cartilage outgrowth during cranial development. Dev Biol. 2004;273:134–148. doi: 10.1016/j.ydbio.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Abzhanov A, Protas M, Grant BR, Grant PR, Tabin CJ. Bmp4 and morphological variation of beaks in Darwin’s finches. Science. 2004;305:1462–1465. doi: 10.1126/science.1098095. [DOI] [PubMed] [Google Scholar]
- Alberch P, Gould SJ, Oster GF, Wake DB. Size and shape in ontogeny and phylogeny. Paleobiology. 1979;5:296–317. [Google Scholar]
- Albrecht UEG, Helms JA, Lin H. Visualization of gene expression patterns by in situ hybridization. In: Daston GP, editor. Molecular and Cellular Methods in Developmental Toxicology. Boca Raton, FL: CRC Press; 1997. pp. 23–48. [Google Scholar]
- Andres G. Untersuchungen an Chimären von Triton und Bombinator. Genetica. 1949;24:387–534. [Google Scholar]
- Ashique AM, Fu K, Richman JM. Signalling via type IA and type IB bone morphogenetic protein receptors (BMPR) regulates intramembranous bone formation, chondrogenesis and feather formation in the chicken embryo. Int J Dev Biol. 2002;46:243–253. doi: 10.1387/ijdb.011535. [DOI] [PubMed] [Google Scholar]
- Atchley WR. Genetic components of size and shape. II. Multivariate covariance patterns in the rat and mouse skull. Evolution. 1981;35:1037–1055. doi: 10.1111/j.1558-5646.1981.tb04973.x. [DOI] [PubMed] [Google Scholar]
- Atchley WR, Hall BK. A model for development and evolution of complex morphological structures. Biol Rev Cam Philos Soc. 1991;66:101–157. doi: 10.1111/j.1469-185x.1991.tb01138.x. [DOI] [PubMed] [Google Scholar]
- Balling R, Mutter G, Gruss P, Kessel M. Craniofacial abnormalities induced by ectopic expression of the homeobox gene Hox-1.1 in transgenic mice. Cell. 1989;58:337–347. doi: 10.1016/0092-8674(89)90848-9. [DOI] [PubMed] [Google Scholar]
- Barlow AJ, Francis-West PH. Ectopic application of recombinant BMP-2 and BMP-4 can change patterning of developing chick facial primordia. Development. 1997;124:391–398. doi: 10.1242/dev.124.2.391. [DOI] [PubMed] [Google Scholar]
- Barlow AJ, Bogardi JP, Ladher R, Francis-West PH. Expression of chick Barx-1 and its differential regulation by FGF-8 and BMP signaling in the maxillary primordia. Dev Dyn. 1999;214:291–302. doi: 10.1002/(SICI)1097-0177(199904)214:4<291::AID-AJA2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Bee J, Thorogood P. The role of tissue interactions in the skeletogenic differentiation of avian neural crest cells. Dev Biol. 1980;78:47–66. doi: 10.1016/0012-1606(80)90317-6. [DOI] [PubMed] [Google Scholar]
- Bell DM, Leung KK, Wheatley SC, Ng LJ, Zhou S, Ling KW, Sham MH, Koopman P, Tam PP, Cheah KS. SOX9 directly regulates the type-II collagen gene. Nat Genet. 1997;16:174–178. doi: 10.1038/ng0697-174. [DOI] [PubMed] [Google Scholar]
- Benson RH, Chapman RE, Siegel AF. On the measurement of morphology and its change. Paleobiology. 1982;8:328–339. [Google Scholar]
- Bobick BE, Thornhill TM, Kulyk WM. Fibroblast growth factors 2, 4, and 8 exert both negative and positive effects on limb, frontonasal, and mandibular chondrogenesis via MEK-ERK activation. J Cell Physiol. 2007;211:233–243. doi: 10.1002/jcp.20923. [DOI] [PubMed] [Google Scholar]
- Bookstein FL. The Measurement of Biological Shape and Shape Change. New York: Springer-Verlag; 1978. [Google Scholar]
- Bookstein FL. Multivariate methods. In: Rohlf FJ, Bookstein FL, editors. Proceedings of the Michgan Morphometrics Workshop, Special Publication Number 2. Ann Arbor, MI: University of Michigan Museum of Zoology; 1990. pp. 75–76. [Google Scholar]
- Chapman RE. Conventional procrustes approaches. In: Rohlf FJ, Bookstein FL, editors. Proceedings of the Michigan Morphometrics Workshop, Special Publication No. 2 . Ann Arbor, MI: The University of Michigan Museum of Zoology; 1990. pp. 251–267. [Google Scholar]
- Coppinger R, Schneider R. Evolution of working dogs. In: Serpell J, editor. The Domestic Dog. Cambridge, UK: Cambridge University Press; 1995. pp. 21–47. [Google Scholar]
- Couly G, Creuzet S, Bennaceur S, Vincent C, Le Douarin NM. Interactions between Hox-negative cephalic neural crest cells and the foregut endoderm in patterning the facial skeleton in the vertebrate head. Development. 2002;129:1061–1073. doi: 10.1242/dev.129.4.1061. [DOI] [PubMed] [Google Scholar]
- Creuzet S, Couly G, Vincent C, Le Douarin NM. Negative effect of Hox gene expression on the development of the neural crest-derived facial skeleton. Development. 2002;129:4301–4313. doi: 10.1242/dev.129.18.4301. [DOI] [PubMed] [Google Scholar]
- Crump JG, Maves L, Lawson ND, Weinstein BM, Kimmel CB. An essential role for Fgfs in endodermal pouch formation influences later craniofacial skeletal patterning. Development. 2004;131:5703–5716. doi: 10.1242/dev.01444. [DOI] [PubMed] [Google Scholar]
- David NB, Saint-Etienne L, Tsang M, Schilling TF, Rosa FM. Requirement for endoderm and FGF3 in ventral head skeleton formation. Development. 2002;129:4457–4468. doi: 10.1242/dev.129.19.4457. [DOI] [PubMed] [Google Scholar]
- de Beer GR. Embryology and Evolution. Oxford, UK: Clarendon Press; 1930. [Google Scholar]
- de Beer GR. The Development of the Vertebrate Skull. Chicago, IL: University of Chicago Press; 1937. [Google Scholar]
- de Crombrugghe B, Lefebvre V, Behringer RR, Bi W, Murakami S, Huang W. Transcriptional mechanisms of chondrocyte differentiation. Matrix Biol. 2000;19:389–394. doi: 10.1016/s0945-053x(00)00094-9. [DOI] [PubMed] [Google Scholar]
- Depew MJ, Lufkin T, Rubenstein JL. Specification of jaw subdivisions by Dlx genes. Science. 2002;298:381–385. doi: 10.1126/science.1075703. [DOI] [PubMed] [Google Scholar]
- Dunlop LL, Hall BK. Relationships between cellular condensation, preosteoblast formation and epithelial-mesenchymal interactions in initiation of osteogenesis. Int J Dev Biol. 1995;39:357–371. [PubMed] [Google Scholar]
- Eames BF, Schneider RA. Quail-duck chimeras reveal spatiotemporal plasticity in molecular and histogenic programs of cranial feather development. Development. 2005;132:1499–1509. doi: 10.1242/dev.01719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eames BF, de la Fuente L, Helms JA. Molecular ontogeny of the skeleton. Birth Defects Res Part C Embryo Today. 2003;69:93–101. doi: 10.1002/bdrc.10016. [DOI] [PubMed] [Google Scholar]
- Eames BF, Sharpe PT, Helms JA. Hierarchy revealed in the specification of three skeletal fates by Sox9 and Runx2. Dev Biol. 2004;274:188–200. doi: 10.1016/j.ydbio.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Eames BF, Allen N, Young J, Kaplan A, Helms JA, Schneider RA. Skeletogenesis in the swell shark Cephaloscyllium ventriosum. J Anat. 2007;210:542–554. doi: 10.1111/j.1469-7580.2007.00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekanayake S, Hall BK. The in vivo and in vitro effects of bone morphogenetic protein-2 on the development of the chick mandible. Int J Dev Biol. 1997;41:67–81. [PubMed] [Google Scholar]
- Ferguson CA, Tucker AS, Sharpe PT. Temporospatial cell interactions regulating mandibular and maxillary arch patterning. Development. 2000;127:403–412. doi: 10.1242/dev.127.2.403. [DOI] [PubMed] [Google Scholar]
- Foppiano S, Hu D, Marcucio RS. Signaling by bone morphogenetic proteins directs formation of an ectodermal signaling center that regulates craniofacial development. Dev Biol. 2007;312:103–114. doi: 10.1016/j.ydbio.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DW, Kaesler RL. Shape analysis: Ideas from the Ostracoda. In: McKinney ML, editor. Heterochrony in Evolution: A Multidisciplinary Approach. New York: Plenum Press; 1988. pp. 53–69. [Google Scholar]
- Francis-West PH, Tatla T, Brickell PM. Expression patterns of the bone morphogenetic protein genes Bmp-4 and Bmp-2 in the developing chick face suggest a role in outgrowth of the primordia. Dev Dyn. 1994;201:168–178. doi: 10.1002/aja.1002010207. [DOI] [PubMed] [Google Scholar]
- Francis-West P, Ladher R, Barlow A, Graveson A. Signalling interactions during facial development. Mechanisms of Development. 1998;75:3–28. doi: 10.1016/s0925-4773(98)00082-3. [DOI] [PubMed] [Google Scholar]
- Francis-West PH, Robson L, Evans DJ. Craniofacial development: the tissue and molecular interactions that control development of the head. Adv Anat Embryol Cell Biol. 2003;169:III–VI. 1–138. doi: 10.1007/978-3-642-55570-1. [DOI] [PubMed] [Google Scholar]
- Garrod DR. Specific inductive flypaper. BioEssays. 1986;5:172–173. doi: 10.1002/bies.950050408. [DOI] [PubMed] [Google Scholar]
- Gendron-Maguire M, Mallo M, Zhang M, Gridley T. Hoxa-2 mutant mice exhibit homeotic transformation of skeletal elements derived from cranial neural crest. Cell. 1993;75:1317–1331. doi: 10.1016/0092-8674(93)90619-2. [DOI] [PubMed] [Google Scholar]
- Gould SJ. Allometry and size in ontogeny and phylogeny. Biological Review. 1966;41:587–640. doi: 10.1111/j.1469-185x.1966.tb01624.x. [DOI] [PubMed] [Google Scholar]
- Gould SJ. Ontogeny and Phylogeny. Cambridge, MA: Harvard University Press; 1977. [Google Scholar]
- Govindarajan V, Overbeek PA. FGF9 can induce endochondral ossification in cranial mesenchyme. BMC Dev Biol. 2006;6:7. doi: 10.1186/1471-213X-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammatopoulos GA, Bell E, Toole L, Lumsden A, Tucker AS. Homeotic transformation of branchial arch identity after Hoxa2 overexpression. Development. 2000;127:5355–5365. doi: 10.1242/dev.127.24.5355. [DOI] [PubMed] [Google Scholar]
- Haldane JBS. On Being the Right Size and Other Essays. Oxford, UK: Oxford University Press; 1985. [Google Scholar]
- Hall BK. Tissue interactions and the initiation of osteogenesis and chondrogenesis in the neural crest-derived mandibular skeleton of the embryonic mouse as seen in isolated murine tissues and in recombinations of murine and avian tissues. J Embryol Exp Morphol. 1980;58:251–264. [PubMed] [Google Scholar]
- Hall BK. The induction of neural crest-derived cartilage and bone by embryonic epithelia: an analysis of the mode of action of an epithelial-mesenchymal interaction. J Embryol Exp Morphol. 1981;64:305–320. [PubMed] [Google Scholar]
- Hall BK. The role of tissue interactions in the growth of bone. In: Dixon AD, Sarnat BG, editors. Factors and Mechanisms Influencing Bone Growth. New York: Alan R. Liss; 1982. pp. 205–215. [Google Scholar]
- Hall BK. Developmental processes underlying heterochrony as an evolutionary mechanism. Can J Zool. 1984;62:1–7. [Google Scholar]
- Hall BK. The role of movement and tissue interactions in the development and growth of bone and secondary cartilage in the clavicle of the embryonic chick. J Embryol Exp Morphol. 1986;93:133–152. [PubMed] [Google Scholar]
- Hall BK. Tissue interactions in the development and evolution of the vertebrate head. In: Maderson PFA, editor. Developmental and Evolutionary Aspects of the Neural Crest. New York: John Wiley & Sons; 1987. pp. 215–260. [Google Scholar]
- Hall BK. Bones and cartilage: developmental and evolutionary skeletal biology. San Diego, CA: Elsevier Academic Press; 2005. [Google Scholar]
- Hamburger V. A Manual of Experimental Embryology. Chicago, IL: The University of Chicago Press; 1942. [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Hammer Ø, Harper DAT. Paleontological Data Analysis. Malden, MA: Blackwell; 2006. [Google Scholar]
- Hanken J, Hall BK. The Skull. Chicago, IL: University of Chicago Press; 1993. [Google Scholar]
- Havens BA, Rodgers B, Mina M. Tissue-specific expression of Fgfr2b and Fgfr2c isoforms, Fgf10 and Fgf9 in the developing chick mandible. Arch Oral Biol. 2006;51:134–145. doi: 10.1016/j.archoralbio.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Healy C, Uwanogho D, Sharpe PT. Expression of the chicken Sox9 gene marks the onset of cartilage differentiation. Ann New York Acad Sci. 1996;785:261–262. doi: 10.1111/j.1749-6632.1996.tb56278.x. [DOI] [PubMed] [Google Scholar]
- Healy C, Uwanogho D, Sharpe PT. Regulation and role of Sox9 in cartilage formation. Dev Dyn. 1999;215:69–78. doi: 10.1002/(SICI)1097-0177(199905)215:1<69::AID-DVDY8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Hu D, Marcucio RS, Helms JA. A zone of frontonasal ectoderm regulates patterning and growth in the face. Development. 2003;130:1749–1758. doi: 10.1242/dev.00397. [DOI] [PubMed] [Google Scholar]
- Hunt P, Clarke JD, Buxton P, Ferretti P, Thorogood P. Stability and plasticity of neural crest patterning and branchial arch Hox code after extensive cephalic crest rotation. Dev Biol. 1998;198:82–104. doi: 10.1006/dbio.1998.8886. [DOI] [PubMed] [Google Scholar]
- Huxley JS. Problems of Relative Growth. London, UK: Methuen; 1932. [Google Scholar]
- Iwamoto M, Kitagaki J, Tamamura Y, Gentili C, Koyama E, Enomoto H, Komori T, Pacifici M, Enomoto-Iwamoto M. Runx2 expression and action in chondrocytes are regulated by retinoid signaling and parathyroid hormone-related peptide (PTHrP) Osteoarthritis Cartilage. 2003;11:6–15. doi: 10.1053/joca.2002.0860. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y, Agathon A, Alexander J, Thisse C, Waldron S, Yelon D, Thisse B, Stainier DY. casanova encodes a novel Sox-related protein necessary and sufficient for early endoderm formation in zebrafish. Genes Dev. 2001;15:1493–1505. doi: 10.1101/gad.892301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Miller CT, Kruze G, Ullmann B, BreMiller RA, Larison KD, Snyder HC. The shaping of pharyngeal cartilages during early development of the zebrafish. Dev Biol. 1998;203:245–263. doi: 10.1006/dbio.1998.9016. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ullmann B, Walker C, Wilson C, Currey M, Phillips PC, Bell MA, Postlethwait JH, Cresko WA. Evolution and development of facial bone morphology in threespine sticklebacks. Proc Natl Acad Sci USA. 2005;102:5791–5796. doi: 10.1073/pnas.0408533102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg CP, Spence JR. Heterochrony and allometry: Lessons from the water strider genus Limnoporus. Evolution. 1993;47:1834–1853. doi: 10.1111/j.1558-5646.1993.tb01273.x. [DOI] [PubMed] [Google Scholar]
- Lande R. Quantitative genetic analysis of multivariate evolution, applied to brain:body size allometry. Evolution. 1979;33:402–416. doi: 10.1111/j.1558-5646.1979.tb04694.x. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Dieterlen-Lievre F, Teillet M. Quail-chick transplantations. In: Bronner-Fraser M, editor. Methods in Avian Embryology. Vol. 51. San Diego, CA: Academic Press; 1996. pp. 23–59. [PubMed] [Google Scholar]
- Liu W, Selever J, Murali D, Sun X, Brugger SM, Ma L, Schwartz RJ, Maxson R, Furuta Y, Martin JF. Threshold-specific requirements for Bmp4 in mandibular development. Dev Biol. 2005;283:282–293. doi: 10.1016/j.ydbio.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Lufkin T, Mark M, Hart C, Dollé P, Lemeur M, Chambon P. Homeotic transformation of the occipital bones of the skull by ectopic expression of a homeobox gene. Nature. 1992;359:835–841. doi: 10.1038/359835a0. [DOI] [PubMed] [Google Scholar]
- Lwigale PY, Schneider RA. Other chimeras: quail-duck and mouse-chick. Methods Cell Biol. 2008;87:59–74. doi: 10.1016/S0091-679X(08)00203-3. [DOI] [PubMed] [Google Scholar]
- Mandler M, Neubuser A. FGF signaling is required for initiation of feather placode development. Development. 2004;131:3333–3343. doi: 10.1242/dev.01203. [DOI] [PubMed] [Google Scholar]
- Marcucio RS, Cordero DR, Hu D, Helms JA. Molecular interactions coordinating the development of the forebrain and face. Dev Biol. 2005;284:48–61. doi: 10.1016/j.ydbio.2005.04.030. [DOI] [PubMed] [Google Scholar]
- McKinney ML. Classifying heterochrony: allometry, size, and time. In: McKinney ML, editor. Heterochrony in Evolution: A Multidisciplinary Approach. New York: Plenum Press; 1988. pp. 17–34. [Google Scholar]
- Merrill AE, Eames BF, Weston SJ, Heath T, Schneider RA. Mesenchyme-dependent BMP signaling directs the timing of mandibular osteogenesis. Development. 2008;135:1223–1234. doi: 10.1242/dev.015933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CT, Schilling TF, Lee K, Parker J, Kimmel CB. sucker encodes a zebrafish Endothelin-1 required for ventral pharyngeal arch development. Development. 2000;127:3815–3828. doi: 10.1242/dev.127.17.3815. [DOI] [PubMed] [Google Scholar]
- Mina M, Upholt WB, Kollar EJ. Enhancement of avian mandibular chondrogenesis in vitro in the absence of epithelium. Arch Oral Biol. 1994;39:551–562. doi: 10.1016/0003-9969(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Mina M, Gluhak J, Upholt WB, Kollar EJ, Rogers B. Experimental analysis of Msx-1 and Msx-2 gene expression during chick mandibular morphogenesis. Dev Dyn. 1995;202:195–214. doi: 10.1002/aja.1002020211. [DOI] [PubMed] [Google Scholar]
- Mina M, Wang YH, Ivanisevic AM, Upholt WB, Rodgers B. Region- and stage-specific effects of FGFs and BMPs in chick mandibular morphogenesis. Dev Dyn. 2002;223:333–352. doi: 10.1002/dvdy.10056. [DOI] [PubMed] [Google Scholar]
- Mitsiadis TA, Caton J, Cobourne M. Waking-up the sleeping beauty: recovery of the ancestral bird odontogenic program. J Exp Zool B Mol Dev Evol. 2006;306:227–233. doi: 10.1002/jez.b.21094. [DOI] [PubMed] [Google Scholar]
- Mohammadi M, McMahon G, Sun L, Tang C, Hirth P, Yeh BK, Hubbard SR, Schlessinger J. Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science. 1997;276:955–960. doi: 10.1126/science.276.5314.955. [DOI] [PubMed] [Google Scholar]
- Murakami S, Kan M, McKeehan WL, de Crombrugghe B. Up-regulation of the chondrogenic Sox9 gene by fibroblast growth factors is mediated by the mitogen-activated protein kinase pathway. Proc Natl Acad Sci USA. 2000;97:1113–1118. doi: 10.1073/pnas.97.3.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noden DM. The role of the neural crest in patterning of avian cranial skeletal, connective, and muscle tissues. Dev Biol. 1983;96:144–165. doi: 10.1016/0012-1606(83)90318-4. [DOI] [PubMed] [Google Scholar]
- Noden D, Schneider RA. Neural crest cells and the community of plan for craniofacial development: historical debates and current perspectives. In: Saint-Jeannet JP, editor. Neural Crest Induction and Differentiation. Vol. 589. Georgetown, TX: Landes Bioscience; 2006. pp. 1–23. [DOI] [PubMed] [Google Scholar]
- Pasqualetti M, Ori M, Nardi I, Rijli FM. Ectopic Hoxa2 induction after neural crest migration results in homeosis of jaw elements in Xenopus. Development. 2000;127:5367–5378. doi: 10.1242/dev.127.24.5367. [DOI] [PubMed] [Google Scholar]
- Petiot A, Ferretti P, Copp AJ, Chan CT. Induction of chondrogenesis in neural crest cells by mutant fibroblast growth factor receptors. Dev Dyn. 2002;224:210–221. doi: 10.1002/dvdy.10102. [DOI] [PubMed] [Google Scholar]
- Piotrowski T, Nusslein-Volhard C. The endoderm plays an important role in patterning the segmented pharyngeal region in zebrafish (Danio rerio) Dev Biol. 2000;225:339–356. doi: 10.1006/dbio.2000.9842. [DOI] [PubMed] [Google Scholar]
- Qiu M, Bulfone A, Ghattas I, Meneses JJ, Christensen L, Sharpe PT, Presley R, Pedersen RA, Rubenstein JL. Role of the Dlx homeobox genes in proximodistal patterning of the branchial arches: mutations of Dlx-1, Dlx-2, and Dlx-1 and -2 alter morphogenesis of proximal skeletal and soft tissue structures derived from the first and second arches. Dev Biol. 1997;185:165–184. doi: 10.1006/dbio.1997.8556. [DOI] [PubMed] [Google Scholar]
- Raff RA. The Shape of Life: Genes, Development, and the Evolution of Animal Form. Chicago, IL: University of Chicago Press; 1996. [Google Scholar]
- Richman JM, Tickle C. Epithelia are interchangeable between facial primordia of chick embryos and morphogenesis is controlled by the mesenchyme. Dev Biol. 1989;136:201–210. doi: 10.1016/0012-1606(89)90142-5. [DOI] [PubMed] [Google Scholar]
- Richman JM, Tickle C. Epithelial-mesenchymal interactions in the outgrowth of limb buds and facial primordia in chick embryos. Dev Biol. 1992;154:299–308. doi: 10.1016/0012-1606(92)90069-s. [DOI] [PubMed] [Google Scholar]
- Richman JM, Herbert M, Matovinovic E, Walin J. Effect of fibroblast growth factors on outgrowth of facial mesenchyme. Dev Biol. 1997;189:135–147. doi: 10.1006/dbio.1997.8656. [DOI] [PubMed] [Google Scholar]
- Rijli FM, Mark M, Lakkaraju S, Dierich A, Dolle P, Chambon P. A homeotic transformation is generated in the rostral branchial region of the head by disruption of Hoxa-2, which acts as a selector gene. Cell. 1993;75:1333–1349. doi: 10.1016/0092-8674(93)90620-6. [DOI] [PubMed] [Google Scholar]
- Roth VL. How elephants grow: heterochrony and the calibration of developmental stages in some living and fossil species. J Vertebrate Paleontol. 1984;4:126–145. [Google Scholar]
- Russell ES. Form and Function: A Contribution to the History of Animal Morphology. London, UK: John Murray Publishers; 1916. [Google Scholar]
- Schilling TF. Genetic analysis of craniofacial development in the vertebrate embryo. BioEssays. 1997;19:459–468. doi: 10.1002/bies.950190605. [DOI] [PubMed] [Google Scholar]
- Schneider RA. Neural crest can form cartilages normally derived from mesoderm during development of the avian head skeleton. Dev Biol. 1999;208:441–455. doi: 10.1006/dbio.1999.9213. [DOI] [PubMed] [Google Scholar]
- Schneider RA. Developmental mechanisms facilitating the evolution of bills and quills. J Anat. 2005;207:563–573. doi: 10.1111/j.1469-7580.2005.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider RA. How to tweak a beak: molecular techniques for studying the evolution of size and shape in Darwin’s finches and other birds. BioEssays. 2007;29:1–6. doi: 10.1002/bies.20517. [DOI] [PubMed] [Google Scholar]
- Schneider RA, Helms JA. The cellular and molecular origins of beak morphology. Science. 2003;299:565–568. doi: 10.1126/science.1077827. [DOI] [PubMed] [Google Scholar]
- Schneider RA, Hu D, Rubenstein JL, Maden M, Helms JA. Local retinoid signaling coordinates forebrain and facial morphogenesis by maintaining FGF8 and SHH. Development. 2001;128:2755–2767. doi: 10.1242/dev.128.14.2755. [DOI] [PubMed] [Google Scholar]
- Schowing J. Influence inductrice de l’encéphale embryonnaire sur le développement du crâne chez le Poulet. J Embryol Exp Morphol. 1968;19:9–32. [PubMed] [Google Scholar]
- Shigetani Y, Nobusada Y, Kuratani S. Ectodermally derived FGF8 defines the maxillomandibular region in the early chick embryo: epithelial-mesenchymal interactions in the specification of the craniofacial ectomesenchyme. Dev Biol. 2000;228:73–85. doi: 10.1006/dbio.2000.9932. [DOI] [PubMed] [Google Scholar]
- Siegel AF, Benson RH. A robust comparison of biological shapes. Biometrics. 1982;38:341–350. [PubMed] [Google Scholar]
- Smith KK. Heterochrony revisited: the evolution of developmental sequences. Biol J Linnean Soc. 2001;73:169–186. [Google Scholar]
- Smith KK. Sequence heterochrony and the evolution of development. J Morphol. 2002;252:82–97. doi: 10.1002/jmor.10014. [DOI] [PubMed] [Google Scholar]
- Smith KK. Time’s arrow: heterochrony and the evolution of development. Int J Dev Biol. 2003;47:613–621. [PubMed] [Google Scholar]
- Smith KK, Schneider RA. Have gene knockouts caused evolutionary reversals in the mammalian first arch? BioEssays. 1998;20:245–255. doi: 10.1002/(SICI)1521-1878(199803)20:3<245::AID-BIES8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Smith MM, Hall BK. Development and evolutionary origins of vertebrate skeletogenic and odontogenic tissues. Biol Rev Cam Philos Soc. 1990;65:277–373. doi: 10.1111/j.1469-185x.1990.tb01427.x. [DOI] [PubMed] [Google Scholar]
- Thompson DAW. On Growth and Form. Cambridge, UK: Cambridge University Press; 1917. [Google Scholar]
- Thorogood P. Mechanisms of morphogenetic specification in skull development. In: Wolff JR, Severs J, Berry M, editors. Mesenchymal-Epithelial Interactions in Neural Development. Berlin, Germany: Springer-Verlag; 1987. pp. 141–152. [Google Scholar]
- Thorogood P. The developmental specification of the vertebrate skull. Development. 1988;103:141–153. doi: 10.1242/dev.103.Supplement.141. [DOI] [PubMed] [Google Scholar]
- Thorogood P. Differentiation and morphogenesis of cranial skeletal tissues. In: Hanken J, Hall BK, editors. The Skull. Vol. 1. Chicago, IL: University of Chicago Press; 1993. pp. 112–152. [Google Scholar]
- Thorogood P, Bee J, Mark Kvd. Transient expression of collagen type II at epitheliomesenchymal interfaces during morphogenesis of the cartilaginous neurocranium. Dev Biol. 1986;116:497–509. doi: 10.1016/0012-1606(86)90150-8. [DOI] [PubMed] [Google Scholar]
- Tosney KW. The segregation and early migration of cranial neural crest cells in the avian embryo. Dev Biol. 1982;89:13–24. doi: 10.1016/0012-1606(82)90289-5. [DOI] [PubMed] [Google Scholar]
- Tucker AS, Lumsden A. Neural crest cells provide species-specific patterning information in the developing branchial skeleton. Evol Dev. 2004;6:32–40. doi: 10.1111/j.1525-142x.2004.04004.x. [DOI] [PubMed] [Google Scholar]
- Tucker AS, Yamada G, Grigoriou M, Pachnis V, Sharpe PT. Fgf-8 determines rostral-caudal polarity in the first branchial arch. Development. 1999;126:51–61. doi: 10.1242/dev.126.1.51. [DOI] [PubMed] [Google Scholar]
- Tyler MS. Epithelial influences on membrane bone formation in the maxilla of the embryonic chick. Anat Rec. 1978;192:225–233. doi: 10.1002/ar.1091920203. [DOI] [PubMed] [Google Scholar]
- Tyler MS. Development of the frontal bone and cranial meninges in the embryonic chick: an experimental study of tissue interactions. Anat Rec. 1983;206:61–70. doi: 10.1002/ar.1092060108. [DOI] [PubMed] [Google Scholar]
- Veitch E, Begbie J, Schilling TF, Smith MM, Graham A. Pharyngeal arch patterning in the absence of neural crest. Curr Biol. 1999;9:1481–1484. doi: 10.1016/s0960-9822(00)80118-9. [DOI] [PubMed] [Google Scholar]
- Wagner G. Untersuchungen an Bombinator-Triton-Chimaeren. Roux’ Archiv für Entwicklungsmechanik der Organismen. 1959;151:136–158. doi: 10.1007/BF00576376. [DOI] [PubMed] [Google Scholar]
- Wall NA, Hogan BL. Expression of bone morphogenetic protein-4 (BMP-4), bone morphogenetic protein-7 (BMP-7), fibroblast growth factor-8 (FGF-8) and sonic hedgehog (SHH) during branchial arch development in the chick. Mech Dev. 1995;53:383–392. doi: 10.1016/0925-4773(95)00453-x. [DOI] [PubMed] [Google Scholar]
- Wang YH, Rutherford B, Upholt WB, Mina M. Effects of BMP-7 on mouse tooth mesenchyme and chick mandibular mesenchyme. Dev Dyn. 1999;216:320–335. doi: 10.1002/(SICI)1097-0177(199912)216:4/5<320::AID-DVDY2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Wassersug R. A procedure for differential staining of cartilage and bone in whole formalin-fixed vertebrates. Stain Technol. 1976;51:131–134. doi: 10.3109/10520297609116684. [DOI] [PubMed] [Google Scholar]
- Wilson J, Tucker AS. Fgf and Bmp signals repress the expression of Bapx1 in the mandibular mesenchyme and control the position of the developing jaw joint. Dev Biol. 2004;266:138–150. doi: 10.1016/j.ydbio.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Wu P, Jiang TX, Suksaweang S, Widelitz RB, Chuong CM. Molecular shaping of the beak. Science. 2004;305:1465–1466. doi: 10.1126/science.1098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Jiang TX, Shen JY, Widelitz RB, Chuong CM. Morphoregulation of avian beaks: comparative mapping of growth zone activities and morphological evolution. Dev Dyn. 2006;235:1400–1412. doi: 10.1002/dvdy.20825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Sohal GS. Embryonic origin of skeletal muscle cells in the iris of the duck and quail. Cell Tissue Res. 1987;249:31–37. doi: 10.1007/BF00215415. [DOI] [PubMed] [Google Scholar]
- Yan YL, Miller CT, Nissen RM, Singer A, Liu D, Kirn A, Draper B, Willoughby J, Morcos PA, Amsterdam A, et al. A zebrafish sox9 gene required for cartilage morphogenesis. Development. 2002;129:5065–5079. doi: 10.1242/dev.129.21.5065. [DOI] [PubMed] [Google Scholar]
- Yoshida CA, Furuichi T, Fujita T, Fukuyama R, Kanatani N, Kobayashi S, Satake M, Takada K, Komori T. Core-binding factor beta interacts with Runx2 and is required for skeletal development. Nat Genet. 2002;32:633–638. doi: 10.1038/ng1015. [DOI] [PubMed] [Google Scholar]
- Zelditch M. Geometric Morphometrics for Biologists: A Primer. Amsterdam, The Netherlands: Elsevier Academic Press; 2004. [Google Scholar]
- Zhao Q, Eberspaecher H, Lefebvre V, De Crombrugghe B. Parallel expression of Sox9 and Col2a1 in cells undergoing chondrogenesis. Dev Dyn. 1997;209:377–386. doi: 10.1002/(SICI)1097-0177(199708)209:4<377::AID-AJA5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]