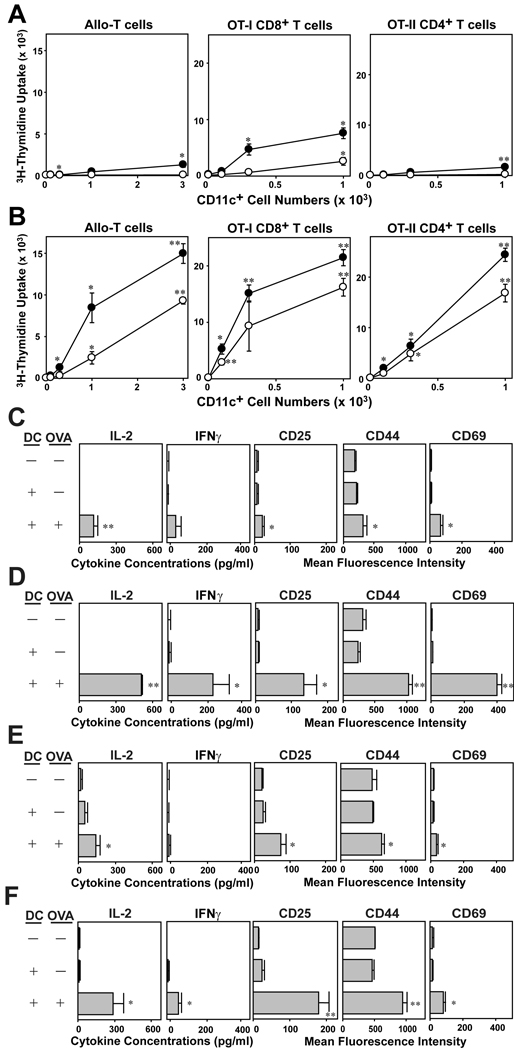
Figure 5.
T cell-stimulatory potentials of DC generated with IL-33. BM cells procured from C57BL/6 mice were cultured for 7 days in the presence of 10 ng/ml IL-33 (A, C, E) or 10 ng/ml GM-CSF (B, D, F). (A, B) After 24 h of additional culture in the presence (closed circles) or absence (open circles) of 10 ng/ml LPS, CD11c+ cells were FACS-purified and cocultured with allogeneic CD3+ T cells purified from BALB/c mice, CD8+ T cells purified from OT-I Tg mice, or CD4+ T cells purified from OT-II Tg mice. CD11c+ cells were added at the indicated cell numbers to micro-cultures of responder T cells (5 × 104 cells/well). To test the ability to activate OT-I and OT-II T cells, CD11c+ cells were pulsed for 1 h with OVA257–264 peptide (10 µg/ml) or OVA323–339 peptide (10 µg/ml) and washed extensively before addition to T cell micro-cultures. Data shown mean ± SD (n = 3) of 3H-thymidine uptake on day 3. *p<0.05, **p<0.01; compared to the levels of proliferation of allogeneic T cells in the absence of CD11c+ cells or the base line levels of OT-I and OT-II T cells stimulated by CD11c+ cells in the absence of OVA peptide pulsing. (C–F) FACS-purified CD11c+ cells from the above BM cultures were pulsed with relevant OVA peptide or PBS alone and then co-cultured with OT-I T cells for 24 h (C, D) or with OT-II T cells for 60 h (E, F). Culture supernatants were then tested for the indicated cytokines, and CD3+ T cells were examined for surface expression of the indicated activation markers. Data show mean ± SD (n = 3). *p< 0.05, **p<0.01; compared to the control panels in which T cells were co-cultured with DC in the absence of OVA peptide pulsing.