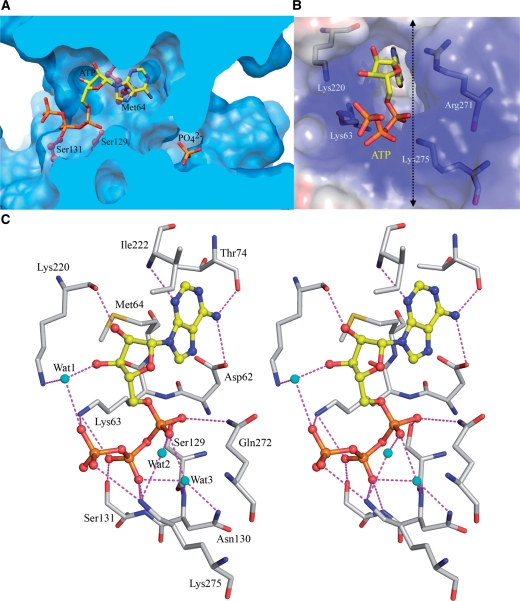
Figure 3.
The active site of HlAIPT. (A) Molecular surface of HlAIPT. The enzyme forms a central reaction channel. The cross-sectional surface, which is cut at the dashed line in (B), is colored light blue. The ball-stick model shows the side chains of Met64, Ser129 and Ser131. The ATP molecule and phosphate ion are shown as rods. (B) A cluster of positively charged residues surround the open end of the ATP-binding channel. (C) Stereoscopic view of ATP binding to the HlAIPT. Hydrogen bonds and charge–charge interactions are shown as dashed lines.