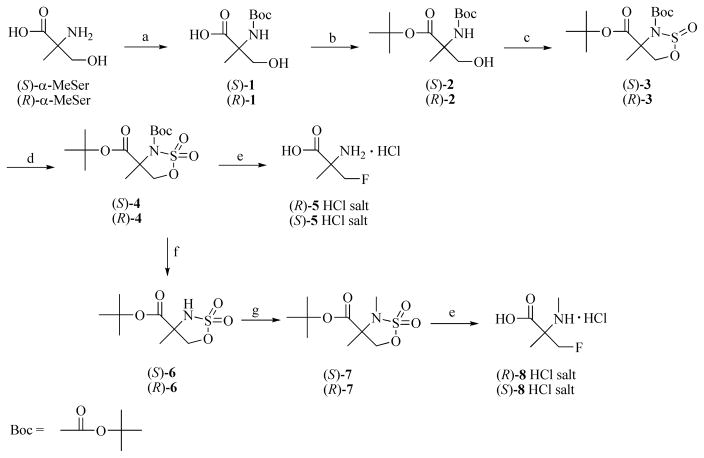
Scheme 1. a. Syntheses of labeling precursors (S)- and (R)-4 and (S)- and (R)-7, and syntheses of the fluorine-19 amino acids (R)- and (S)-5 and (R)- and (S)-8.
a Reagents and conditions: a. (Boc)2O, MeOH-Et3N-NaOH (aq.), rt. b. (CH3)2NCH[OC(CH3)3]2, 80–90 °C. c. SOCl2, Pyr, −40 °C. d. NaIO4, RuCl3, CH3CN/H2O, 0 °C to rt. e. n-Bu4NF, r.t. then 3N HCl, 85 °C. f. MeSO3H, t-BuOAc, DCM, rt. g. Me2SO4, NaH, THF, rt.