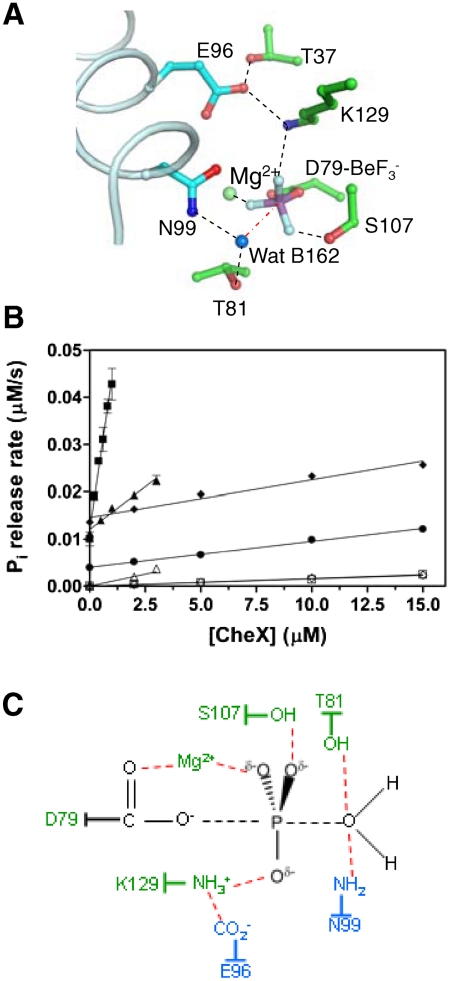
Fig. 3.
Active site structure and kinetic characterization of CheX and CheY3. (A) Close-up view of the active site. Residues are green for CheY3 and cyan for CheX. The ordered water molecule (Wat B162) is a blue sphere and Mg2+ is a light green sphere. Hydrogen bonds revealed by the structure are represented by black-dashed lines. The red-dashed line between Wat B162 and the beryllium atom (distance of 3.4 Å) is nearly collinear with the bond connecting the beryllium atom and Asp79 OD1. (B) CheX phosphatase activities. The CheY3 concentration is 15 μM. Reactions contained wild-type CheX/wild-type CheY3 (closed squares), CheX 96EA/wild-type CheY3 (closed triangles), CheX 99NA/wild-type CheY3 (closed diamonds), or wild-type CheX/ CheY3 81TA (closed circles). Rates measured in the absence of CheY3 for wild-type CheX (open squares), CheX 96EA (open triangles) and CheX 99NA (open diamonds) reflects nonspecific phosphatase activity due to trace contaminants, and was subtracted out. (C) Schematic model for transition state stabilization in CheX -catalyzed dephosphorylation of CheY3. The bipyramidal transition state is colored black with dashed lines representing partial bonds. Stabilizing interactions are represented with red-dashed lines with the assumption that interactions observed in 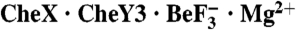 persist in the transition state. One of several possible hydrogen bonding arrangements between CheY3 T81 or CheX N99 and the water is shown. Residues from CheY3 (green) and CheX (cyan) are indicated.
persist in the transition state. One of several possible hydrogen bonding arrangements between CheY3 T81 or CheX N99 and the water is shown. Residues from CheY3 (green) and CheX (cyan) are indicated.