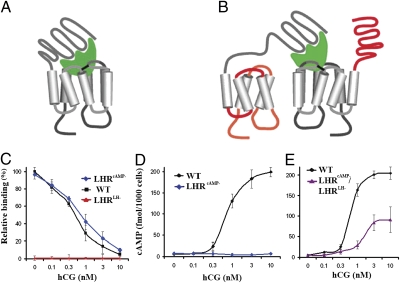
Fig. 1.
Demonstration of intermolecular cooperation and di/oligomerization on binding- and signaling-deficient LHR mutants in cultured cells. (A and B) Schematic presentation of intramolecular (cis) and intermolecular (trans) activation of GPCRs. (A) When a hormone (green) binds to its WT receptor, the occupied receptor activates itself to generate a signal(s) (5). (B) Alternatively, a GPCR complexed with hormone may activate another GPCR molecule, as evidenced by intermolecular activation of signaling-deficient mutant (LHRcAMP− with red connecting loops) by binding-deficient mutant (LHRLH− with red extracellular domain) when both mutants are coexpressed in a cell (33, 35, 36). (C–E) Cell culture experiments on ligand binding and cAMP generation of the LHR mutants. (C) Binding-deficient receptor (LHRLH−; red line) was incapable of displaying specific binding of [125I]-hCG in the presence of increasing concentrations of unlabeled hCG (0–10 nM). In contrast, WT (black line) and signaling-deficient LHR (LHRcAMP−; blue line) bound [125I]-hCG specifically and with similar apparent affinity. (D) WT LHR (black line) produced cAMP in response to hCG stimulation. However, neither the signaling-deficient (LHRcAMP−, blue line) nor the binding-deficient (LHRLH−, superimposed with the former) mutant produced cAMP. (E) When both (LHRLH− and LHRcAMP−) mutants were coexpressed in HEK-293 cells, cAMP production was partially restored in response to hCG (purple line) as compared to WT LHR (black line). One of three experiments with similar results transfecting with BAC-LHR clones is shown. Each point is the mean ± SD of triplicate incubations. Experiments with cDNA clones produced similar results.